Enthalpy Change (Δh) Measurement: Techniques And Applications
To determine the enthalpy change (ΔH) of a chemical reaction, one can employ various methods. Calorimetry, by directly measuring the heat released or absorbed during the reaction, is a common approach. Hess’s Law allows for the calculation of ΔH for complex reactions by combining the enthalpy changes of individual steps. Additionally, standard enthalpy of formation, combustion, and reaction can be utilized to determine ΔH for specific types of reactions, such as formation of new compounds or combustion processes.
Enthalpy Change: Unveiling the Secrets of Thermodynamics
In the realm of thermodynamics, enthalpy change plays a pivotal role, shedding light on the intricate dance of energy within chemical reactions. Imagine it as a measure of energy absorbed or released as substances undergo a transformation. It’s like a balance sheet, tracking the energy flow during these captivating molecular gymnastics.
Enthalpy is a thermodynamic property that captures both the internal energy of a system and the work it can perform on its surroundings. And when this enthalpy changes, we enter the fascinating world of enthalpy change (ΔH). ΔH quantifies the net energy transfer during a chemical reaction, revealing whether it’s an energy-absorbing (endothermic) or energy-releasing (exothermic) affair.
Endothermic reactions are like thirsty sponges, eagerly soaking up energy from their surroundings. They require an input of heat to push the reaction forward, creating new substances with higher potential energy. Think of a bonfire on a cold winter night, where the flames draw heat from the air to fuel their fiery dance.
In contrast, exothermic reactions are generous energy providers, releasing heat into their surroundings as they proceed. They’re like miniature powerhouses, generating energy as bonds are broken and new ones are formed. Imagine a burning candle, casting a warm glow into the darkness. The heat it radiates is a testament to the exothermic nature of the combustion reaction.
Understanding enthalpy change is essential for deciphering the intricate dynamics of chemical reactions. It’s a key player in determining the feasibility, spontaneity, and direction of these transformations. Join us on this captivating journey into the world of enthalpy change, where we’ll unravel the mysteries and uncover the secrets of energy flow in a dynamic universe.
Enthalpy Change (ΔH): Understanding the Energy Flow in Chemical Reactions
In the realm of chemistry, understanding the flow of energy during chemical reactions is crucial. Enthalpy change (ΔH) plays a pivotal role in this energy exchange, providing valuable insights into the behavior of chemical systems. It measures the heat absorbed or released by a reaction at constant pressure and is an indicator of the reaction’s energetic favorability.
Enthalpy, denoted by the symbol H, is a state function that represents the total thermal energy of a system. It encompasses both internal energy (the energy within the molecules) and external work (the work done by or on the system against external forces).
ΔH, the change in enthalpy, quantifies the net energy exchanged between a system and its surroundings during a chemical reaction. A positive ΔH indicates an endothermic reaction, where heat is absorbed from the surroundings to break bonds and create new ones. In contrast, a negative ΔH signifies an exothermic reaction, releasing heat into the surroundings as bonds are formed.
Understanding the difference between endothermic and exothermic reactions is crucial. Endothermic reactions require an input of energy to overcome the activation energy barrier, which is the minimum energy required to initiate the reaction. Conversely, exothermic reactions release energy as bonds are formed, making them more likely to occur spontaneously.
Exothermic and Endothermic Reactions: Understanding the Heat Exchange
In the realm of chemistry, reactions can be categorized into two distinct types based on their heat exchange: exothermic and endothermic. These reactions exhibit contrasting behaviors that profoundly influence the processes they participate in.
Exothermic Reactions: Releasing Energy into the Environment
Exothermic reactions, as their name suggests, *release energy into the surroundings* during their course. This energy typically manifests as heat, causing the reaction mixture to become warmer. A classic example of an exothermic reaction is the combustion of methane, where methane and oxygen *react to form carbon dioxide and water with the release of heat*.
Endothermic Reactions: Absorbing Energy from the Environment
In contrast to exothermic reactions, endothermic reactions *absorb energy from the surroundings* during their progression. This energy is required to break the chemical bonds of the reactants, resulting in a *cooler reaction mixture*. A prominent illustration of an endothermic reaction is the electrolysis of water, where water is *split into hydrogen and oxygen with the absorption of heat*.
Characteristics of Exothermic and Endothermic Reactions
Distinguishing between exothermic and endothermic reactions is crucial for predicting their behavior and potential applications. Here’s a table summarizing their key characteristics:
| Characteristic | Exothermic Reaction | Endothermic Reaction |
|---|---|---|
| Energy Exchange | Releases heat | Absorbs heat |
| Temperature Change | Increases | Decreases |
| Spontaneity | More spontaneous | Less spontaneous |
| Examples | Combustion, neutralization | Melting, photosynthesis |
Applications and Relevance
Exothermic and endothermic reactions play vital roles in various fields. Exothermic reactions are harnessed for energy production, such as in the combustion of fuels in engines and the production of heat in chemical hand warmers. Endothermic reactions, on the other hand, are utilized in processes like cooling systems, refrigeration, and the production of certain chemicals.
Understanding the nature of exothermic and endothermic reactions not only enhances our comprehension of chemical reactions but also enables us to design processes and technologies that effectively utilize these reactions for various applications.
Enthalpy Change: A Comprehensive Guide
Heat of Reaction
Unveiling the Energetic Dance of Chemical Change
In the realm of thermodynamics, the heat of reaction reignites as the captivating protagonist, orchestrating the energetic drama that unfolds during chemical transformations. This measure of heat energy, intricately intertwined with the enthalpy change (ΔH), reveals the symphony of energy exchange that accompanies the creation and destruction of chemical bonds.
The Heat Equation: Unraveling the Positive and Negative
The heat of reaction, a numerical value measured in units of kilojoules per mole (kJ/mol), exists in a realm of its own. It portrays the energy absorbed or released during a reaction, providing a testament to the chemical choreography at play. A positive heat of reaction, like a burst of fireworks, signifies an exothermic reaction, where the products possess less energy than the reactants. The system releases energy into its surroundings, warming them in the process.
Conversely, an endothermic reaction absorbs energy from its surroundings, leaving them slightly cooler. Like a magnet seeking its opposite, the products in an endothermic reaction crave more energy than the reactants, leading to a negative heat of reaction. This energy deficit, like a hungry flame, is siphoned from the environment, creating a cooling effect.
Navigating the Energetic Landscape
The heat of reaction serves as a compass, guiding us through the labyrinthine world of chemical reactions. Exothermic reactions release energy, making them energetically favorable and often spontaneous. Endothermic reactions, on the other hand, require external energy input, hindering spontaneity. Understanding the heat of reaction empowers us to predict the likelihood of a reaction occurring under specific conditions.
Calorimetry: Unveiling the Secrets of Heat Changes
In the realm of thermodynamics, understanding enthalpy change (ΔH) is paramount. But how do we measure these elusive changes? Enter calorimetry, a scientific technique that holds the key to unlocking the secrets of heat during chemical reactions.
The Principles of Calorimetry
Calorimetry is based on the principle that every change in temperature is accompanied by a transfer of heat. By measuring the heat transferred during a chemical reaction, we can indirectly determine the enthalpy change. This is achieved using a device called a calorimeter.
Calorimeters: Tools for Measuring Heat
Calorimeters come in various forms, but the basic principle remains the same. They consist of an insulated container that prevents heat from escaping and a thermometer to measure temperature changes. As a chemical reaction proceeds inside the calorimeter, the heat released or absorbed by the reaction is transferred to the calorimeter and its contents.
Determining ΔH Using Calorimetry
The heat transferred during a reaction can be quantified using the equation:
Q = mCΔT
where:
- Q is the heat transferred in joules
- m is the mass of the calorimeter and its contents in kilograms
- C is the specific heat capacity of the calorimeter and its contents in joules per gram per Kelvin
- ΔT is the change in temperature in Kelvin
By measuring the mass, specific heat capacity, and temperature change, we can calculate the heat transferred during the reaction. Once we have Q, we can determine ΔH using the relationship:
ΔH = -Q
Endothermic vs. Exothermic Reactions
If the heat transferred is positive (Q > 0), the reaction is endothermic. This means that the reaction absorbs heat from the surroundings, causing the temperature to decrease. In contrast, if the heat transferred is negative (Q < 0), the reaction is exothermic. In this case, the reaction releases heat to the surroundings, leading to an increase in temperature.
Hess’s Law: Unraveling the Enthalpy Mystery
Imagine you’re a culinary chemist trying to create a mouthwatering dish. You carefully select your ingredients, each with its unique enthalpy change—the amount of heat absorbed or released during a reaction. To predict the total enthalpy change of your culinary masterpiece, you’ll need to master the secrets of Hess’s Law.
Hess’s Law is a chemical superpower that empowers you to calculate the overall enthalpy change of a complex reaction by summing the enthalpy changes of individual steps. It’s like assembling a chemical puzzle, where each piece (reaction step) has its own unique enthalpy signature.
Let’s say you’re creating a delicious crème brûlée. You start by heating sugar in a pan and melting it to form caramel. This endothermic reaction absorbs heat from its surroundings, represented by a positive ΔH. Your next step is to mix the melted caramel with eggs and milk. This exothermic step releases heat, characterized by a negative ΔH. Finally, you bake the custard, another exothermic step.
Using Hess’s Law, you can calculate the total enthalpy change of your crème brûlée adventure. Simply add up the enthalpy changes for each step: ΔHmelting + ΔHmixing + ΔHbaking = ΔHtotal.
This clever law allows you to determine whether your crème brûlée will tantalize your taste buds with a warm embrace (exothermic) or a cooling sensation (endothermic). Isn’t that magical?
Standard Enthalpy of Formation: Delving into Reactions’ Energy Dynamics
In the realm of thermodynamics, the standard enthalpy of formation is a pivotal concept that unlocks insights into chemical reactions. Enthalpy itself represents the total energy content of a system, encompassing both internal energy and the energy associated with its volume.
The standard enthalpy of formation refers specifically to the enthalpy change that occurs when one mole of a compound is formed from its constituent elements at standard conditions (temperature of 298 K and pressure of 1 atm). By convention, the standard enthalpy of formation of elementary substances (e.g., O₂ or H₂) is defined as zero.
This concept plays a crucial role in calculating the enthalpy change, ΔH, for reactions involving the formation of new compounds. ΔH represents the energy released (exothermic) or absorbed (endothermic) during a chemical reaction.
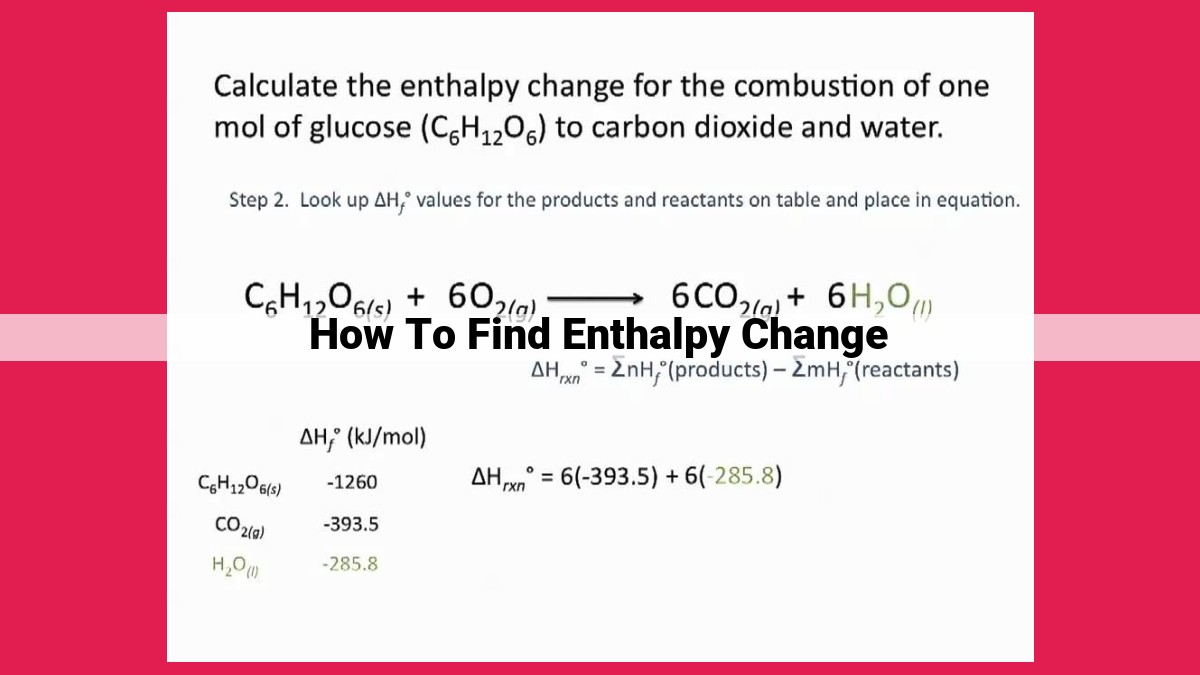
Consider the example of the combustion of methane (CH₄) to form carbon dioxide (CO₂) and water (H₂O):
CH₄ + 2O₂ → CO₂ + 2H₂O
Using standard enthalpies of formation, ΔH for this reaction can be determined as follows:
ΔH = [Σ (Standard Enthalpy of Formation of Products)] - [Σ (Standard Enthalpy of Formation of Reactants)]
Plugging in the values from a table of standard enthalpies of formation, we get:
ΔH = [(-393.5 kJ/mol) + (-285.8 kJ/mol)] - [(-74.8 kJ/mol) + (0 kJ/mol)]
ΔH = -802.6 kJ/mol
The negative ΔH indicates that this reaction is exothermic, releasing 802.6 kJ of energy per mole of methane burned.
By understanding the concept of standard enthalpy of formation, chemists can predict the energy changes associated with chemical reactions, gaining valuable insights into their feasibility and spontaneity.
Unveiling the Secrets of Standard Enthalpy of Combustion
In the realm of thermodynamics, standard enthalpy of combustion reigns supreme as a measure of the energy released during the complete burning of a substance. This enigmatic concept holds the key to predicting the fiery dance of combustion reactions.
When a fuel undergoes combustion, it undergoes a vigorous exothermic reaction with oxygen, releasing an abundance of heat energy. This exothermic nature is reflected in the negative value associated with the standard enthalpy of combustion, signifying the energy liberated into the surroundings.
The standard enthalpy of combustion is a precious tool for chemists, allowing them to forecast the energy output of various fuels. By comparing the standard enthalpies of combustion of different substances, we can determine which ones hold the most potential energy and thus make the most efficient fuels.
Moreover, the standard enthalpy of combustion provides insights into the thermodynamic feasibility of combustion reactions. A negative standard enthalpy of combustion indicates that the reaction is spontaneous and will proceed with the release of heat, while a positive standard enthalpy of combustion suggests that the reaction is nonspontaneous and requires an input of energy to occur.
In summary, the standard enthalpy of combustion is an indispensable tool for understanding the energy dynamics of combustion reactions. It allows us to predict the energy released, evaluate the feasibility of reactions, and make informed choices about the fuels we use. By harnessing this knowledge, we can optimize energy production, conserve resources, and unlock the transformative power of combustion.
Standard Enthalpy of Reaction: Predicting the Energetic Fate of Reactions
Understanding the energetic behavior of chemical reactions is crucial in various fields of science and engineering. The standard enthalpy of reaction (ΔH°) is a fundamental thermodynamic parameter that provides valuable information about the feasibility and spontaneity of a reaction under standard conditions (298 K and 1 atm).
ΔH° represents the heat change that accompanies a reaction when all reactants and products are in their standard states, which typically refer to pure substances at 1 atm and 298 K. A positive ΔH° indicates an endothermic reaction, meaning that the reaction absorbs heat from the surroundings. Conversely, a negative ΔH° signifies an exothermic reaction,释放ing heat into the surroundings.
The standard enthalpy of reaction can be used to predict the spontaneity of a reaction under standard conditions. According to the second law of thermodynamics, spontaneous reactions tend to proceed with a decrease in free energy (ΔG). Therefore, for a reaction to be spontaneous, ΔG must be negative. The relationship between ΔG and ΔH° is given by the equation:
ΔG = ΔH° - TΔS°
where ΔS° is the standard entropy change of the reaction and T is the temperature in Kelvin. Under standard conditions, a negative ΔH° suggests a positive ΔG, indicating that the reaction is nonspontaneous. On the other hand, a negative ΔH° and a positive ΔS° can result in a negative ΔG, indicating a spontaneous reaction.
Determining the standard enthalpy of reaction is essential for understanding the energetics of chemical processes. It allows scientists to predict the direction and extent of reactions, evaluate their feasibility, and design strategies for optimizing reaction conditions. Moreover, ΔH° provides insights into the stability and reactivity of compounds, aiding in the development of new materials and technologies.