Electron Transport Chain: Generating Atp Through Oxidative Phosphorylation
-
Primary Role of the Electron Transport Chain:
- The primary role of the electron transport chain (ETC) is to generate ATP through oxidative phosphorylation, a process that couples electron transfer with energy production.
-
Oxidative Phosphorylation:
- Electrons transferred along the ETC create a proton gradient across the mitochondrial membrane.
- This gradient drives ATP synthesis by ATP synthase, the final enzyme in the ETC.
Primary Role of the Electron Transport Chain:
- Explain the electron transport chain’s involvement in oxidative phosphorylation and energy production.
The Electron Transport Chain: The Powerhouse of Cellular Energy Production
In the captivating realm of cellular biology, the electron transport chain reigns supreme as the conductor of life’s symphony. Its intricate choreography generates the energy that fuels our every movement, thought, and heartbeat. Prepare to delve into the hidden depths of this enigmatic molecular orchestra and unravel the secrets of its incredible power.
The Electron Transport Chain: Maestro of Oxidative Phosphorylation
Like a skilled maestro orchestrating a symphony, the electron transport chain is the heart of a process known as oxidative phosphorylation. Here, electron carriers, such as NADH and FADH2, surrender their precious electrons, initiating a cascade of energy-rich events. These electrons dance along a series of protein complexes, like acrobats on a high wire, releasing their energy in controlled bursts.
Proton Motive Force: Creating an Electrical Divide
As electrons leap from one complex to the next, their journey creates an electrical divide. This gradient of hydrogen ions, or protons, across the mitochondrial membrane is the conductor of a symphony of its own. The protons, like eager performers, seek a way to cross over, creating a surge of energy that drives the final act of this cellular drama.
ATP Synthase: The Energy Converters
Behold the ATP synthase, the master conductor of energy conversion. Its FoF1 complex acts like a revolving door, allowing protons to cascade through its channels. This controlled flow drives the synthesis of ATP, the molecular currency of energy that fuels our cells. With each ATP molecule produced, the symphony of cellular life continues unabated.
Oxidative Stress: The Potential Pitfall
As with any performance, there are potential risks. The electron transport chain, in its rush to generate energy, can inadvertently produce reactive oxygen species (ROS), the disruptive vandals of cellular machinery. To ensure a harmonious balance, nature has deployed antioxidants as the vigilant protectors of our cells, neutralizing these ROS and preserving the integrity of our cellular orchestra.
Oxidative Phosphorylation: The Powerhouse of the Cell
Embark on a Journey into the Electron Transport Chain
Picture a tiny cellular factory humming with activity – the mitochondrion. This powerhouse orchestrates a crucial process known as oxidative phosphorylation, which generates the energy that fuels our very existence. At its heart lies the electron transport chain, a complex symphony of proteins that dance electrons along a delicate pathway.
The Dance of Electrons
The electron transport chain, residing in the mitochondrial membrane, is a series of protein complexes that resemble stepping stones in a river. Electrons, like eager travelers, flow down this cascade, from high-energy donors to low-energy acceptors. These donors, such as NADH and FADH2, carry electrons from glucose breakdown. Oxygen, the ultimate electron acceptor, stands ready at the end of the chain to receive these tiny charge-carriers.
The Proton Gradient: A Reservoir of Power
As electrons waltz through the transport chain, they don’t travel alone. They also drive the creation of a proton (hydrogen ion) gradient across the mitochondrial membrane. This gradient, known as the proton motive force, is a reservoir of potential energy, akin to a dam holding back a river’s might.
ATP Synthase: Harnessing the Proton Power
Seizing the energy stored in the proton gradient is ATP synthase, a remarkable protein complex embedded in the mitochondrial membrane. Like a tiny turbine, it spins as protons rush through it, drawing their energy to synthesize ATP – the universal currency of cellular energy. This process, akin to a mini power plant, transforms the proton gradient into a steady flow of ATP, powering the myriad biochemical reactions that sustain life.
Oxidative Stress: A Cautionary Tale
While the electron transport chain is a vital energy source, it also harbors a potential danger. As electrons dance along the chain, a handful of them can escape, forming reactive oxygen species (ROS) – highly reactive molecules that can damage cellular components. To mitigate this risk, the cell employs antioxidant defenses, like valiant knights protecting a castle, to neutralize these rogue electrons and maintain cellular harmony.
The electron transport chain and oxidative phosphorylation are intricate mechanisms that provide the energy for life’s processes. By harnessing the flow of electrons and creating a proton gradient, these cellular powerhouses generate ATP, the fuel that drives our cells and empowers us to live and thrive. Understanding these processes not only illuminates the fundamental principles of biology but also provides insights into metabolic disorders and other conditions where energy production goes awry.
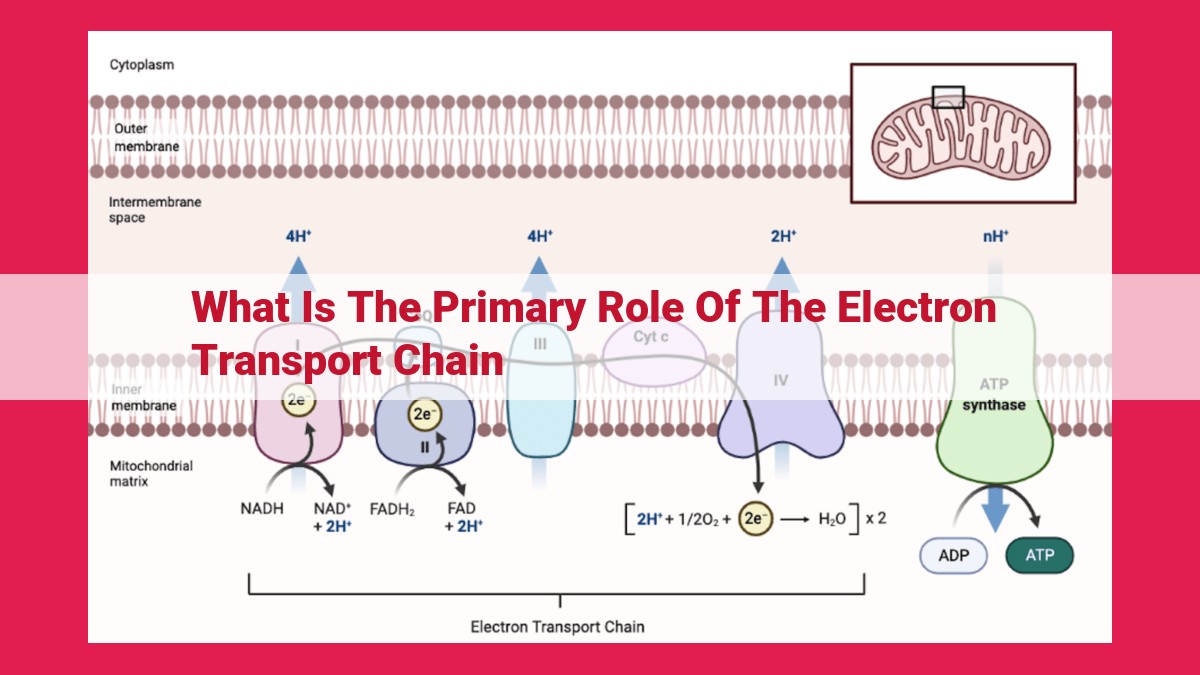
Electron Transport Chain (ETC):
- Describe the structure and function of the ETC, including its protein complexes and electron transfer mechanisms.
- Highlight the electron donors (NADH, FADH2) and the electron acceptor (oxygen).
The Electron Transport Chain: The Powerhouse of Cellular Energy Production
In the realm of cellular energy, the electron transport chain (ETC) stands as a central player, generating the bulk of the energy that fuels our cellular processes. Let’s embark on a storytelling journey to unravel its intricate structure and vital functions.
The Protein Complexes and Electron Highway
Picture the ETC as a bustling highway, lined with protein complexes that serve as waystations for electrons. These proteins, NADH-dehydrogenase, succinate-dehydrogenase, cytochrome c reductase, and cytochrome c oxidase, act as pumps and carriers, facilitating the transfer of electrons along the chain like a relay race.
At the beginning of the highway, NADH and FADH2, the hardworking electron donors, hand off their energy-rich electrons to the first protein complex. Oxygen, the ultimate electron acceptor, waits at the other end, eager to receive the final batch.
Oxidative Phosphorylation: The Energy Gold Mine
As electrons flow through the ETC, they lose energy, which is used to pump hydrogen ions (protons) across the mitochondrial membrane. This creates a proton gradient, a reservoir of energy that’s ready to be tapped.
In the final leg of the journey, electrons combine with protons and oxygen to form water. Simultaneously, protons rush back across the membrane through a channel called ATP synthase. This surge of protons drives the synthesis of ATP, the primary energy currency of our cells.
Oxidative Stress: A Delicate Balance
While the ETC is a vital energy producer, it also poses a potential threat. As electrons flow through the chain, reactive oxygen species (ROS) are produced as a byproduct. These ROS can damage cellular components if not kept in check. Fortunately, our bodies possess antioxidants, which act as guardians against oxidative stress.
The Heart of Cellular Energy
The electron transport chain is the beating heart of cellular energy production. It converts the energy stored in NADH and FADH2 into a usable form, ATP. This energy powers a myriad of cellular processes, from muscle contraction to brain function. Without the ETC, our cells would grind to a halt, leaving us without the energy to sustain life.
So, let’s appreciate the electron transport chain, the tireless worker that keeps our cellular engines humming and empowers us to live our energy-filled lives.
Electron Donors and Acceptors: Fueling the Electron Transport Chain
The electron transport chain (ETC) is a crucial player in cellular energy production. Just like a conveyor belt, it carries electrons along a series of protein complexes, generating energy along the way. These electrons don’t appear out of thin air; they have to come from somewhere, and that’s where electron donors step in.
The primary electron donors for the ETC are two molecules: NADH and FADH2. NADH (nicotinamide adenine dinucleotide) and FADH2 (flavin adenine dinucleotide) are coenzymes derived from the breakdown of glucose during cellular respiration. Remember, glucose is the sugar that our bodies use for energy.
NADH and FADH2 carry electrons that have been acquired during the Krebs (citric acid) cycle and glycolysis. When these electrons are passed onto the ETC, it’s like adding fuel to the fire. The electrons provide the energy that drives the ETC and ultimately leads to ATP production.
Now, let’s meet the final electron acceptor: oxygen (O2). Oxygen is the ultimate recipient of these electrons. It plays a pivotal role in the ETC, as it combines with electrons and protons to form water. This process is essential for completing the electron transfer chain and providing a final destination for the electrons.
Proton Motive Force:
- Explain the mechanism by which electron transfer creates a gradient of hydrogen ions (protons) across the mitochondrial membrane.
The Electron Transport Chain: A Cellular Powerhouse
Tucked away within the mitochondria of eukaryotic cells lies a molecular marvel known as the electron transport chain (ETC). This intricate network of proteins plays a vital role in the production of ATP, the energy currency that fuels our cells. Through a series of orchestrated electron transfers, the ETC harnesses the energy released from the breakdown of glucose to generate this essential molecule.
Oxidative Phosphorylation: The Power of Electrons
At the heart of the ETC’s function lies a process called oxidative phosphorylation. As electrons cascade down the ETC, they pass through a series of protein complexes that serve as energy-transducing machines. These complexes pump hydrogen ions (protons) across the mitochondrial membrane, creating an electrochemical gradient known as the proton motive force.
Building the Proton Motive Force
The relentless movement of electrons along the ETC establishes a Proton Motive Force—a concentration gradient of protons across the mitochondrial membrane. This gradient acts as a driving force, providing the energy needed to power the synthesis of ATP.
ATP Synthase: The Energy Converter
Embedded in the mitochondrial membrane is an enzyme complex known as ATP synthase. This molecular maestro harnesses the power of the proton motive force to create ATP from adenosine diphosphate (ADP) and inorganic phosphate. As protons flow down their concentration gradient through ATP synthase, they drive the rotation of a central stalk, which catalyzes the formation of ATP.
The Rewards of Electron Transfer
The electron transport chain serves as the primary generator of ATP in our cells. This precious molecule provides the energy required for a myriad of cellular processes, from muscle contraction to nerve impulses. The ETC’s ability to convert chemical energy into a usable form is essential for life itself.
Oxidative Stress: The Dark Side of Electron Transfer
As electrons navigate the ETC, there is an inevitable byproduct—the generation of reactive oxygen species (ROS). These molecules can damage cellular components, leading to oxidative stress. Fortunately, our cells are equipped with antioxidants that neutralize ROS, protecting our delicate machinery from harm.
Unveiling the Powerhouse of Energy: The Electron Transport Chain and ATP Synthase
In the heart of our cells, a microscopic symphony unfolds, orchestrating the production of energy that fuels our every action. This energy-producing saga unfolds within the electron transport chain (ETC), a vital component of cellular respiration.
The Electron Transport Chain: The Energy Generator
The ETC, a series of protein complexes embedded in the membrane of mitochondria, plays a pivotal role in oxidative phosphorylation, the process of generating ATP, the primary energy currency of cells. As electrons are transported along the chain, they lose energy, which is harnessed to pump protons across the mitochondrial membrane. This proton gradient creates a proton motive force that drives the synthesis of ATP.
Oxidative Phosphorylation: The Dance of Protons and Electrons
Oxidative phosphorylation is an intricate ballet of electron transfer and proton pumping. Electrons donated by NADH and FADH2, molecules generated during cellular respiration, embark on a journey through the ETC. As electrons cascade down the chain, proton pumps embedded within the complexes propel protons across the membrane, creating an electrochemical gradient.
ATP Synthase: The ATP-Generating Maestro
ATP synthase, a molecular machine residing in the mitochondrial membrane, is the mastermind behind ATP synthesis. Its H+ channel, Fo, allows protons to down the concentration gradient, driving the rotation of a central stalk, F1. This rotational motion catalyzes the synthesis of ATP from ADP and inorganic phosphate.
Energy Production: The Ultimate Goal
The electron transport chain and ATP synthase are synergistic partners, orche_strating energy production_. The ETC generates the proton motive force, and ATP synthase harnesses this force to create ATP. This ATP is the fuel for cellular activities, powering everything from muscle contraction to brain function.
Oxidative Stress: The Byproduct of Energy Generation
Electron transfer within the ETC can result in the production of reactive oxygen species (ROS), potentially harmful molecules. However, our cells possess antioxidants, which neutralize ROS and protect against oxidative damage.
The electron transport chain and ATP synthase are the powerhouses of our cells, generating the energy that underpins our very existence. This complex machinery is a testament to the sophistication and beauty of cellular processes. By understanding the intricate workings of these components, we gain a deeper appreciation for the fundamental processes that sustain life.
Unveiling the Electron Transport Chain: Nature’s Powerhouse for Cellular Energy
In the bustling metropolis of the cell, there lies a remarkable energy-generating machinery known as the electron transport chain (ETC). Like a tireless worker, the ETC toils away, extracting energy from nutrients and converting it into the cell’s primary energy source, adenosine triphosphate (ATP).
The ETC plays a pivotal role in oxidative phosphorylation, the process of generating ATP through the transfer of electrons along a series of protein complexes embedded in the inner mitochondrial membrane. This intricate arrangement of proteins resembles a miniature electrical grid, with electrons flowing from one complex to another like tiny currents.
One of the key players in this energy-generating machinery is the proton motive force. As electrons pass through the ETC, they release energy, which is used to pump hydrogen ions (protons) across the mitochondrial membrane, creating a concentration gradient. This gradient serves as a powerful reservoir of energy, like a dam storing water.
The ATP synthase complex capitalizes on this energy gradient. Spanning the mitochondrial membrane, this complex acts as a molecular turbine, harnessed by the proton flow. As protons rush down the gradient, they drive the rotation of the turbine, which in turn triggers the synthesis of ATP molecules. This process is akin to a waterwheel spinning machinery to generate electricity.
Energy Production: The Ultimate Goal
The primary role of the ETC is to generate ATP, the universal currency of cellular energy. ATP fuels countless cellular processes, from muscle contraction to nerve impulse transmission. Without this vital energy source, the cell’s machinery would grind to a halt, leaving it a lifeless shell.
Oxidative Stress: A Byproduct of Energy Generation
While the ETC is a powerhouse for energy production, its relentless activity comes with a potential drawback: oxidative stress. As electrons flow through the ETC, some may “escape” and react with oxygen to form reactive oxygen species (ROS), which are highly damaging to cellular molecules. To combat this, cells employ antioxidant defenses to neutralize ROS, ensuring the ETC’s function remains a source of life, not destruction.
Delving into the Electron Transport Chain: Unraveling the Secrets of Cellular Energy and Oxidative Stress
The Electron Transport Chain: The Engine of Cellular Energy
The electron transport chain (ETC) is a vital component of our cells, playing a crucial role in generating the energy that fuels our bodily functions. This process, known as oxidative phosphorylation, involves the transfer of electrons along a series of protein complexes, establishing a proton gradient that drives the synthesis of ATP, the primary energy currency of the cell.
The ETC: A Complex Symphony of Proteins
The ETC is embedded within the inner mitochondrial membrane, composed of four protein complexes: complex I, complex II, complex III, and complex IV. These complexes act as electron carriers, accepting electrons from NADH and FADH2 molecules and passing them along the chain. The final electron acceptor, oxygen, plays a critical role in the ETC by combining with electrons and protons to form water.
Electron Donors and Acceptors: The Fuel for the ETC
NADH and FADH2, generated during glycolysis and the Krebs cycle, donate electrons to the ETC. As electrons flow along the chain from complex to complex, energy is released, creating a proton gradient across the mitochondrial membrane.
Proton Motive Force: The Driving Force of ATP Synthesis
The proton gradient is a key element in the process of oxidative phosphorylation. As protons move back across the membrane through a channel in ATP synthase, a membrane-spanning enzyme complex, the energy released drives the synthesis of ATP from ADP and inorganic phosphate.
Oxidative Stress: A Potential Pitfall
During electron transfer, reactive oxygen species (ROS), such as free radicals, can be produced. These molecules are highly reactive and can cause cellular damage if not controlled. Antioxidants, such as vitamin C and glutathione, play a vital role in neutralizing ROS and protecting cells from oxidative stress.
The electron transport chain is a complex and essential process that generates ATP, providing the energy that powers countless cellular activities. Understanding the ETC and its role in oxidative stress provides a deeper appreciation for the intricate workings of our cells and the importance of maintaining cellular health.