Unveiling The Electron Transport Chain: How Cells Generate Energy And Metabolites
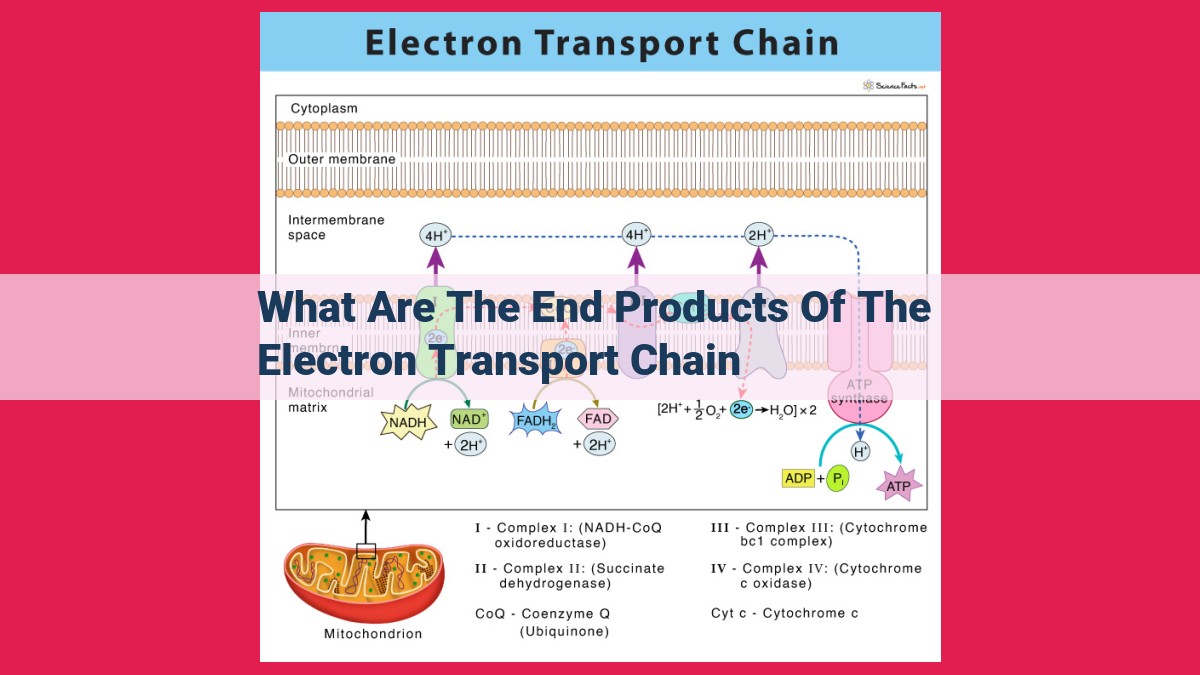
The electron transport chain culminates in the production of essential energy intermediates and byproducts. It generates adenosine triphosphate (ATP), the cellular energy currency, and reduces nicotinamide adenine dinucleotide (NAD+) and flavin adenine dinucleotide (FAD) to NADH and FADH2, respectively, which serve as electron carriers. Additionally, the chain produces water and utilizes oxygen as the terminal electron acceptor, releasing it as a byproduct.
Delving into the Energy Factory: The Electron Transport Chain in Cellular Respiration
Imagine your body as a bustling city, constantly buzzing with activities to sustain life. Within this intricate metropolis, cellular respiration serves as the powerhouse, generating the energy that fuels our every movement and thought. And at the heart of this energy factory lies a critical process called the electron transport chain.
The electron transport chain, like a miniature electrical grid, plays a pivotal role in cellular respiration, the process that converts energy stored in food into usable energy for cells. It’s a series of protein complexes embedded within the inner membrane of mitochondria, the organelles often referred to as the “powerhouses” of cells. These complexes act as electron carriers, passing electrons from one to another, ultimately generating the energy currency of cells: adenosine triphosphate (ATP).
End Products of the Electron Transport Chain:
End Products of the Electron Transport Chain
The electron transport chain (ETC) is a crucial component of cellular respiration, generating energy in the form of ATP. As electrons flow through the ETC, they release energy used to pump protons across a membrane, creating an electrochemical gradient. This gradient drives the synthesis of ATP through a molecular machine called ATP synthase.
The ETC also produces several other notable end products that play vital roles in cellular function:
Adenosine Triphosphate (ATP):
- Composition and Structure: ATP consists of an adenine molecule bonded to a ribose sugar and three phosphate groups.
- Role as Energy Currency: ATP is the primary energy currency of cells, providing quick access to energy for various cellular processes, including muscle contraction, protein synthesis, and transporting molecules across membranes.
Nicotinamide Adenine Dinucleotide Hydrogen (NADH):
- Composition and Structure: NADH is a dinucleotide composed of two nucleotides: nicotinamide adenine dinucleotide (NAD+) bound to two hydrogen atoms.
- Function as an Electron Carrier: NADH carries electrons from glycolysis and the citric acid cycle to the ETC. By donating its electrons, NADH becomes oxidized back to NAD+.
Flavin Adenine Dinucleotide Hydrogen (FADH2):
- Composition and Structure: FADH2 is a flavin derivative bound to an adenine nucleotide.
- Function as an Electron Carrier: Similar to NADH, FADH2 carries electrons from the citric acid cycle to the ETC. It is oxidized back to FAD after donating its electrons.
Water:
- Composition and Structure: Water is a molecule consisting of two hydrogen atoms bonded to an oxygen atom.
- Role as a Byproduct: Water is a product of the electron transfer from NADH and FADH2 to oxygen at the end of the ETC.
Oxygen:
- Composition and Structure: Oxygen is a diatomic molecule consisting of two oxygen atoms.
- Role as a Terminal Electron Acceptor: Oxygen is the final electron acceptor in the ETC, receiving electrons from the electron carriers to form water.
The Powerhouse of the Cell: Understanding Adenosine Triphosphate (ATP)
In the realm of cellular respiration, the electron transport chain plays a pivotal role, acting as the final stage of this intricate process that generates energy for the cell. As electrons pass through the chain, a cascade of reactions unfolds, leading to the production of a crucial molecule: ATP.
ATP: The Energy Currency of Cells
Think of ATP as the cellular equivalent of currency. It’s the primary energy source that fuels all our biological processes, from muscle contractions to chemical reactions. The composition of ATP is simple yet elegant: a molecule of adenine, a nitrogenous base, attached to a chain of three sugar molecules and three phosphate groups.
The phosphate groups are where the magic happens. Each phosphate bond contains a substantial amount of energy. When these bonds are broken, energy is released and immediately available for the cell’s needs. ATP functions like a battery, storing energy and delivering it whenever and wherever it’s required.
The Electron Transport Chain: ATP’s Power Source
The electron transport chain is like a conveyor belt for electrons, transferring them from high-energy states to low-energy states. As the electrons flow through the chain, their energy is harnessed to pump protons across a membrane, creating a concentration gradient.
This gradient provides the driving force for ATP synthesis. A special enzyme called ATP synthase uses the proton gradient to rotate, converting the mechanical energy into chemical energy. This energy is used to synthesize ATP from adenosine diphosphate (ADP) and inorganic phosphate.
ATP is truly the lifeblood of the cell, providing the energy necessary for countless essential processes. Its synthesis through the electron transport chain is a remarkable example of cellular efficiency and precision. By understanding the role of ATP, we gain a deeper appreciation for the intricate machinery that sustains life within us.
Nicotinamide Adenine Dinucleotide Hydrogen (NADH): The Electron Carrier
In the intricate dance of cellular respiration, NADH takes center stage as a crucial electron carrier. This coenzyme, composed of nicotinamide, adenine, and a pair of ribose sugars, plays a pivotal role in glycolysis and the citric acid cycle.
Structure and Composition:
NADH is a dinucleotide, meaning it consists of two nucleotide units joined together. Each nucleotide is made up of a nitrogenous base, a ribose sugar, and a phosphate group. The nicotinamide base is the key component that harbors an electron-carrying pyridine ring.
Electron Carrier in Glycolysis:
During glycolysis, the first stage of cellular respiration, NADH is produced when glyceraldehyde-3-phosphate dehydrogenase enzyme oxidizes glyceraldehyde-3-phosphate to 1,3-bisphosphoglycerate. This reaction involves the transfer of two electrons from the aldehyde group of glyceraldehyde-3-phosphate to NAD+, resulting in the formation of NADH.
Electron Carrier in the Citric Acid Cycle:
NADH’s electron-carrying role continues in the citric acid cycle, where it serves as an electron acceptor for several key reactions. In each of these reactions, dehydrogenase enzymes transfer electrons from their substrates to NAD+, generating NADH. These electrons are then passed along the electron transport chain, ultimately leading to the production of ATP, the cellular energy currency.
Flavin Adenine Dinucleotide Hydrogen (FADH2): The Electron Carrier in the Citric Acid Cycle
In the intricate dance of cellular respiration, the electron transport chain plays a pivotal role, generating ATP, the energy currency of cells. Among the electron carriers involved, Flavin Adenine Dinucleotide Hydrogen (FADH2) stands out as a key player in the citric acid cycle.
FADH2 is a coenzyme composed of a flavin adenine dinucleotide (FAD) molecule attached to two hydrogen atoms. FAD consists of a riboflavin (vitamin B2) group bound to an adenosine monophosphate (AMP) molecule.
Within the citric acid cycle, FADH2 serves as an electron carrier, accepting electrons from succinate, an intermediate molecule. These electrons are then passed on to the electron transport chain, contributing to the generation of ATP.
Unlike Nicotinamide Adenine Dinucleotide Hydrogen (NADH), another electron carrier in the citric acid cycle, FADH2 only donates two electrons to the chain. This difference in electron capacity accounts for the varying amounts of ATP produced from each molecule of NADH and FADH2.
FADH2 is essential for the efficient extraction of energy from glucose during cellular respiration. Its role as an electron carrier enables the transfer of electrons to the electron transport chain, harnessing their energy for the synthesis of ATP. Without FADH2, the citric acid cycle would be significantly less efficient, resulting in reduced energy production.
Therefore, FADH2 plays a crucial role in the interconnected web of cellular respiration, enabling cells to convert nutrients into usable energy to fuel their biological processes.
The Astonishing Byproduct: Uncovering the Role of Water in Cellular Respiration
Embarking on a cellular adventure, let’s delve into the enigmatic world of the electron transport chain. This intricate dance of electrons culminates in the creation of life’s energy currency, ATP. But amidst this symphony, a humble byproduct emerges: water.
Water, the elixir of life, plays a pivotal role in cellular respiration. Its structure, a harmonious embrace of two hydrogen atoms and an oxygen atom, embodies simplicity. Yet, this unassuming molecule holds a profound significance in the electron transport chain.
As electrons gracefully navigate the transport chain, their journey ends with a final encounter with oxygen. This union sparks an extraordinary transformation, releasing energy that fuels the formation of ATP, the fuel for our cells. Simultaneously, water emerges as a byproduct of this biochemical dance.
This seemingly innocuous byproduct is not merely an afterthought; it is a testament to the intricate balance of cellular processes. Water represents the fulfillment of the electron transport chain, carrying away the remnants of the energetic journey. It is a symbol of the meticulousness of life’s machinery, where every step contributes to the symphony of existence.
So, next time you quench your thirst with a sip of water, remember its unassuming beginnings as a byproduct of the electron transport chain. It is a testament to the interconnectedness of life, where even the humblest molecules play a vital role in the grand scheme of cellular existence.
Cellular Respiration’s Powerhouse: Unraveling the Electron Transport Chain’s Secrets
At the heart of every living cell, cellular respiration takes place – a complex dance of chemical reactions that generate energy to power our bodies. Within this intricate process lies the electron transport chain, a vital enzyme complex that orchestrates the final stage of the energy-extraction journey.
As the electrons travel through the electron transport chain, they lose energy, which is harnessed to create adenosine triphosphate (ATP), the primary energy currency of all living cells. This high-energy molecule is the fuel that powers our every thought, movement, and cellular function.
Amongst the electron transport chain’s key players are nicotinamide adenine dinucleotide hydrogen (NADH) and flavin adenine dinucleotide hydrogen (FADH2), electron carriers that transport high-energy electrons from the earlier stages of cellular respiration. These electrons are then passed down the chain, releasing energy that drives proton pumps across the mitochondrial inner membrane.
These proton pumps, like tiny molecular fountains, create a proton gradient – a difference in proton concentration across the membrane. This gradient provides the driving force for the final step in the electron transport chain: the reduction of oxygen.
Oxygen, the terminal electron acceptor in the chain, awaits at the end of this electron-passing cascade. As the electrons reach oxygen, they combine with protons to form water, the humble byproduct of cellular respiration. In this union, the abundant oxygen in our atmosphere plays a crucial role in facilitating the efficient energy extraction from food molecules.
So, as we breathe in oxygen, we are not only providing an essential component for cellular respiration but also enabling the creation of the energy that powers our very existence. The electron transport chain, with its intricate choreography and vital players, stands as a testament to the wonders of life’s chemical machinery.