Unlocking Electron Orbitals: A Comprehensive Guide To Electron Configuration And Energy Distribution
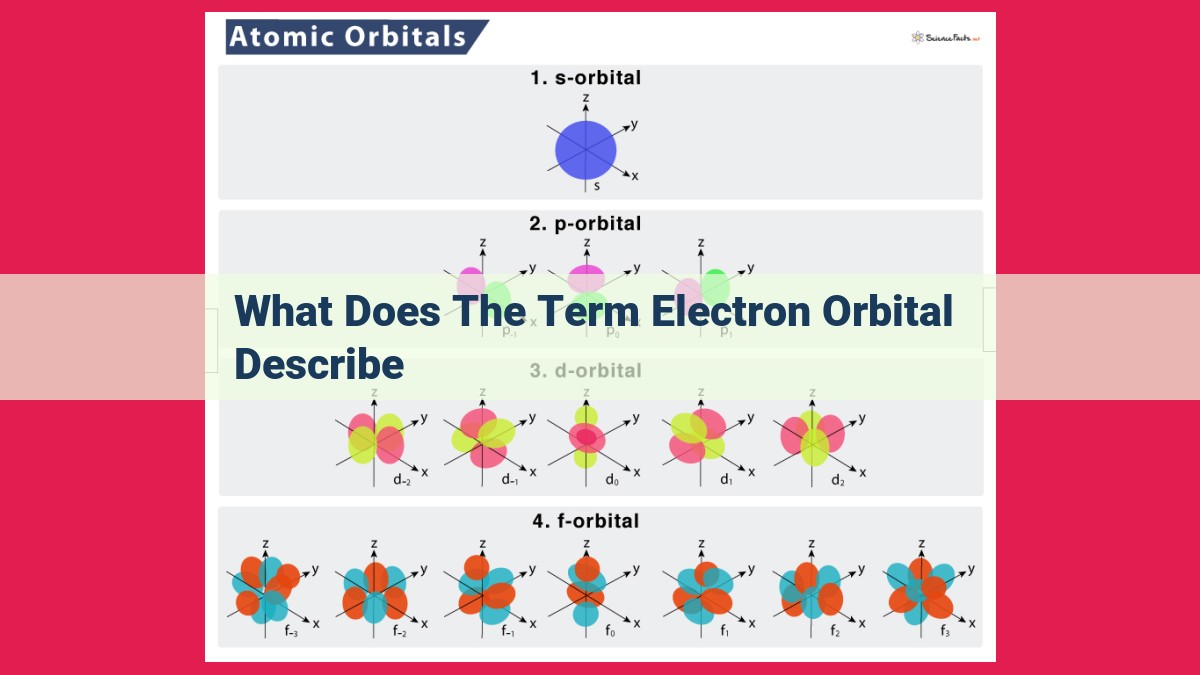
Electron orbitals describe the three-dimensional regions around the atomic nucleus where electrons are most likely to be found. They are defined by a set of quantum numbers, including the principal quantum number (n), angular momentum quantum number (l), and magnetic quantum number (ml), which determine the size, shape, and orientation of the orbital. The electron cloud model is used to represent the distribution of electrons within an orbital, with higher energy orbitals being larger and more diffuse.
Electron Orbitals: Unveiling the Architecture of Atoms
In the realm of atomic structure, electron orbitals play a pivotal role in understanding the arrangement and behavior of electrons. These orbitals, often visualized as three-dimensional regions in space, are the designated areas where electrons reside around the atomic nucleus. Their existence is crucial for deciphering the chemical and physical properties of elements.
What are Electron Orbitals?
Imagine the electron cloud as a dense sphere of negative charge surrounding the nucleus. Within this cloud, electrons occupy specific regions known as orbitals. Each orbital is mathematically described by a wave function that defines the probability of finding an electron within it.
Quantum Numbers: Guiding the Electron’s Path
The behavior of electrons in orbitals is governed by a set of four quantum numbers:
- Principal Quantum Number (n): Indicates the energy level or shell of the orbital.
- Angular Momentum Quantum Number (l): Describes the shape of the orbital (s, p, d, f).
- Magnetic Quantum Number (ml): Specifies the orientation of the orbital in space.
- Spin Quantum Number (ms): Represents the intrinsic spin of the electron (up or down).
Electron Properties: Charge and Mass
Electrons are subatomic particles with an elementary negative charge and a negligible mass compared to protons and neutrons. Their movement and interactions with the nucleus and other electrons shape the chemical and physical properties of atoms.
Electron orbitals provide a framework for comprehending the distribution and behavior of electrons within atoms. By understanding the quantum numbers that govern these orbitals, scientists can predict the characteristics and reactivity of elements. Electron orbitals are the cornerstone of atomic physics and chemistry, providing insights into the fundamental building blocks of our universe.
Electron Orbitals: Delving into the Anatomy of Atoms
Introduction
In the bustling metropolis of an atom, where subatomic particles dance in harmony, electrons are the enigmatic inhabitants that form the foundation of chemical and physical phenomena. Understanding their behavior requires a deep dive into the realm of electron orbitals, the spatial domains where electrons reside. In this blog, we embark on an intriguing journey to unravel the mysteries of atomic orbitals and their influence on the very fabric of matter.
The Electron Cloud and Orbital Shape
Imagine a celestial cloud of negative charge enveloping the nucleus—this ephemeral entity is known as the electron cloud. Within this ethereal expanse, electrons swirl in perpetual motion, occupying distinct regions called atomic orbitals. These orbitals are not physical structures but rather probabilistic descriptions of where electrons are most likely to be found.
Quantum Numbers: Governing the Orbital Landscape
The precise positioning of electrons within orbitals is governed by a set of quantum numbers: the principal quantum number (n), angular momentum quantum number (l), magnetic quantum number (ml), and spin quantum number (ms). These enigmatic numbers provide a comprehensive blueprint for understanding the properties and behaviors of atomic orbitals.
Principal Quantum Number (n): A Tale of Size and Energy
The principal quantum number (n) dictates the size and energy of the electron cloud. Think of it as a hierarchical ladder, with each ascending step representing a larger and more energetic orbital. The higher the n, the farther the electron resides from the nucleus, and the more energy it possesses.
Angular Momentum Quantum Number (l): Shaping the Orbital’s Architecture
The angular momentum quantum number (l) determines the shape of the atomic orbital. It assigns values from 0 to n-1, and each value corresponds to a specific orbital type: s, p, d, f, and so on. These shapes, ranging from the spherical symmetry of s orbitals to the intricate lobes of d and f orbitals, govern the electron’s angular momentum.
Magnetic Quantum Number (ml): Orienting the Orbital in Space
The magnetic quantum number (ml) dictates the orientation of the atomic orbital in space. For a given l value, ml can take values ranging from -l to +l, inclusive. This subtle nuance determines how the orbital is oriented with respect to the magnetic field, influencing the electron’s spatial distribution.
Spin Quantum Number (ms): The Intrinsic Spin
The spin quantum number (ms) captures the intrinsic spin of the electron, a fundamental property that can be either “up” or “down.” This spin, independent of the electron’s motion, plays a crucial role in determining the electron’s magnetic behavior and in obeying the Pauli Exclusion Principle, which stipulates that no two electrons within an atom can have the same set of quantum numbers.
Electron:
- Describe the charge and mass of electrons.
- Relate the electron cloud model to the distribution of electrons in atomic orbitals.
Electrons: The Hidden Architects of Atomic Structure
Electrons are the fundamental building blocks of atoms, possessing a negative charge and an incredibly small mass. These tiny particles, when arranged in specific patterns, determine the chemical and physical properties of every substance in the universe.
Within an atom, electrons reside in a mysterious realm known as orbitals, which are invisible regions where they have the highest probability of being found. Like clouds, these orbitals surround the atom’s nucleus, each with a unique shape and energy level.
The electron cloud model depicts the distribution of electrons within these orbitals. It predicts that electrons can be found anywhere within the orbital’s volume, rather than in fixed, point-like locations. This model provides a probabilistic description of electron behavior, indicating the likelihood of finding electrons in different regions of space.
Unraveling the Enigma of Orbital Shapes: Delving into Quantum Numbers
Introduction:
Electron orbitals, the enigmatic realms where electrons reside within atoms, play a pivotal role in understanding the intricate dance of atomic structure. These ethereal clouds of probability shape the destiny of chemical elements and govern their interactions.
The Electron Cloud: Embracing Uncertainty
Envision an electron cloud as a hazy aura enveloping the atomic nucleus. Within this cloud, electrons dance with an unpredictable nature, their positions ever-shifting. This cloud represents the region where electrons are most likely to be found, a testament to the inherent uncertainty of quantum mechanics.
Enter the Quantum Numbers: Guiding the Electron Symphony
The electron cloud is not a chaotic void but rather a structured symphony governed by a quartet of quantum numbers:
- Principal Quantum Number (n): This number dictates the electron’s energy level and, consequently, the size of its orbital.
- Angular Momentum Quantum Number (l): Like a ballerina’s twirl, this number defines the shape of the electron’s orbital, giving rise to designations such as s, p, d, and f.
- Magnetic Quantum Number (ml): This number reveals the orbital’s orientation in space, determining the direction of its lobes.
- Spin Quantum Number (ms): Each electron possesses an intrinsic spin, either “up” or “down.” This spin distinguishes individual electrons within an orbital.
Unveiling Orbital Shapes: A Dance of Quantum Numbers
The Angular Momentum Quantum Number (l) is the maestro of orbital shapes. Its value determines the geometry of the electron cloud:
- s-orbitals: For l = 0, the electron cloud assumes a spherical shape, symmetrically enveloping the nucleus.
- p-orbitals: With l = 1, the electron cloud adopts dumbbell-shaped p-orbitals, oriented along the x, y, and z axes.
- d-orbitals: Electrons in d-orbitals (l = 2) occupy more complex, cloverleaf-shaped orbitals, with intricate lobes and nodal planes.
- f-orbitals: f-orbitals (l = 3) exhibit even more exotic shapes, with intricate patterns of lobes and nodal surfaces.
The Magnetic Quantum Number (ml) then steps in as the choreographer, dictating the orientations of the orbitals in space. For each l value, ml can take on a range of values that determine the direction of the orbital’s lobes.
Conclusion:
Electron orbitals, governed by a symphony of quantum numbers, are the foundation for deciphering the enigmatic world of atomic structure. Understanding these shapes empowers us to unravel the secrets of chemical bonding and predict the properties of elements, unlocking the mysteries of the molecular world.
Understanding the Principal Quantum Number (n): The Key to Electron Cloud Size and Energy
In our journey toward unraveling the mysteries of the atomic realm, we encounter a fundamental concept that governs the behavior of electrons: the Principal Quantum Number (n). This number holds the key to understanding the size and energy of the electron cloud, a pivotal component in determining the properties of atoms.
The Principal Quantum Number (n) is a positive integer that denotes the energy level or shell in which an electron resides. As n increases, the energy level of the electron also rises. This is because electrons in higher energy levels are farther away from the nucleus, the positively charged core of the atom. The greater the distance from the nucleus, the weaker the electrostatic attraction between the electron and the nucleus, resulting in higher energy.
The size of the electron cloud is directly proportional to the Principal Quantum Number. Electrons move in quantized orbits, forming a cloud of probability around the nucleus. The higher the value of n, the larger the electron cloud becomes. This is because electrons in higher energy levels have greater potential energy, which translates into more space to occupy.
The Principal Quantum Number (n) plays a crucial role in determining the chemical and physical properties of atoms. Atoms with electrons in higher energy levels are more reactive, as they are more easily removed from the atom. Conversely, electrons in lower energy levels are more tightly bound and less likely to participate in chemical reactions.
Furthermore, the Principal Quantum Number (n) has implications for the formation of molecular orbitals and the structure of molecules. The energy difference between different energy levels determines the wavelength of light that can be absorbed or emitted by an atom. This property is exploited in spectroscopy, a powerful technique used to identify and characterize atoms and molecules.
In summary, the Principal Quantum Number (n) is an essential concept in understanding the behavior of electrons in atoms. It dictates the energy level, size, and reactivity of electrons, providing invaluable insights into the fundamental nature of matter.
Angular Momentum Quantum Number (l): The Orbital Shape Determinant
The Angular Momentum Quantum Number, denoted by l, plays a crucial role in shaping the electron cloud within atomic orbitals. It determines the specific shape of an orbital and, consequently, the distribution of electrons within it.
The l value can take integer values ranging from 0 to n-1, where n is the Principal Quantum Number. Each l value corresponds to a specific orbital shape:
- l = 0: s orbital (spherical shape)
- l = 1: p orbital (dumbbell shape with three orientations in space)
- l = 2: d orbital (complex shapes with five orientations)
- l = 3: f orbital (even more complex shapes with seven orientations)
Electron Cloud Distribution
The electron cloud is an abstract representation of the region around the nucleus where electrons are most likely to be found. The l value influences how the electron cloud is distributed within the orbital:
- l = 0: The electron cloud is uniformly distributed in all directions, forming a sphere.
- l = 1: The electron cloud is concentrated in two lobes along the x, y, or z axis.
- l = 2: The electron cloud becomes more complex, with four lobes oriented in the x, y, z, and xy planes.
- l = 3: The electron cloud takes on even more intricate shapes, with six lobes oriented in various directions.
By understanding the Angular Momentum Quantum Number and its impact on orbital shape, scientists can gain insights into the behavior of electrons within atoms. This knowledge forms the foundation for comprehending chemical and physical phenomena at the atomic and molecular levels.
Magnetic Quantum Number (ml):
- Explain how the Magnetic Quantum Number (ml) determines the orientation of atomic orbitals in space.
- Discuss the spatial symmetry of the electron cloud based on ml values.
Magnetic Quantum Number: Directing Orbitals in Space
The magnetic quantum number (ml) is a fascinating aspect of electron orbitals that dictates their orientation in space. It’s like the compass that guides the electron cloud, shaping its distribution and symmetry.
Each orbital can have a specific ml value, which ranges from -l to +l. For example, an orbital with an l value of 1 can have ml values of -1, 0, or +1. These ml values correspond to three different orientations of the orbital in space.
Imagine an orbital as a map of where electrons are likely to be found. The ml value tells us how that map is tilted or twisted relative to the other orbitals in the same energy level. For instance, in p orbitals (l = 1), ml = -1 represents an orbital oriented along the x-axis, ml = 0 corresponds to an orbital along the y-axis, and ml = +1 indicates an orbital along the z-axis.
This spatial symmetry has profound implications for the behavior of electrons within the atom. Orbital interactions and the formation of chemical bonds are all influenced by the orientation of the electron clouds. By understanding ml, we gain insight into the intricate dance of electrons that underpins the very nature of matter.
The Enigmatic World of Electron Orbitals: Delving into the Spin Quantum Number (ms)
In our exploration of the fascinating realm of electron orbitals, we encounter the enigmatic Spin Quantum Number (ms). This elusive property plays a pivotal role in shaping the behavior of electrons within atoms, influencing their orientation and orchestrating the delicate balance of the electron cloud.
The Essence of Electron Spin
Electrons, those tiny particles that whirl around the atomic nucleus, possess an intrinsic property known as spin. Imagine these electrons as tiny spinning tops, each with a magnetic moment—an inherent magnetic field—that arises from their rotation. The Spin Quantum Number (ms) quantifies this spin, assigning it one of two possible values: up (ms = +1/2) or down (ms = -1/2).
The Pauli Exclusion Principle: A Dance of Distinction
The enigmatic Spin Quantum Number, together with the other quantum numbers, governs the behavior of electrons in atomic orbitals. The Pauli Exclusion Principle asserts that no two electrons within an atom can have the exact same set of quantum numbers. This means that each orbital can accommodate a maximum of two electrons, but with opposite spins.
This principle dictates the unique configurations of electrons within orbitals. For instance, in an s orbital, with its spherical shape, two electrons can reside, one with spin up and the other with spin down. This dance of distinction ensures that electrons maintain their individuality, avoiding collisions and paving the way for the intricate chemistry of our world.
The Spin Quantum Number (ms) is an integral component of the electron orbital model. By quantifying electron spin, it shapes the distribution and behavior of electrons within atoms. This understanding lies at the foundation of modern chemistry and physics, enabling us to unravel the mysteries of atomic structure and the fascinating phenomena that govern our universe.