Electron And Molecular Geometry: Understanding The Spatial Distribution Of Electrons And Molecules
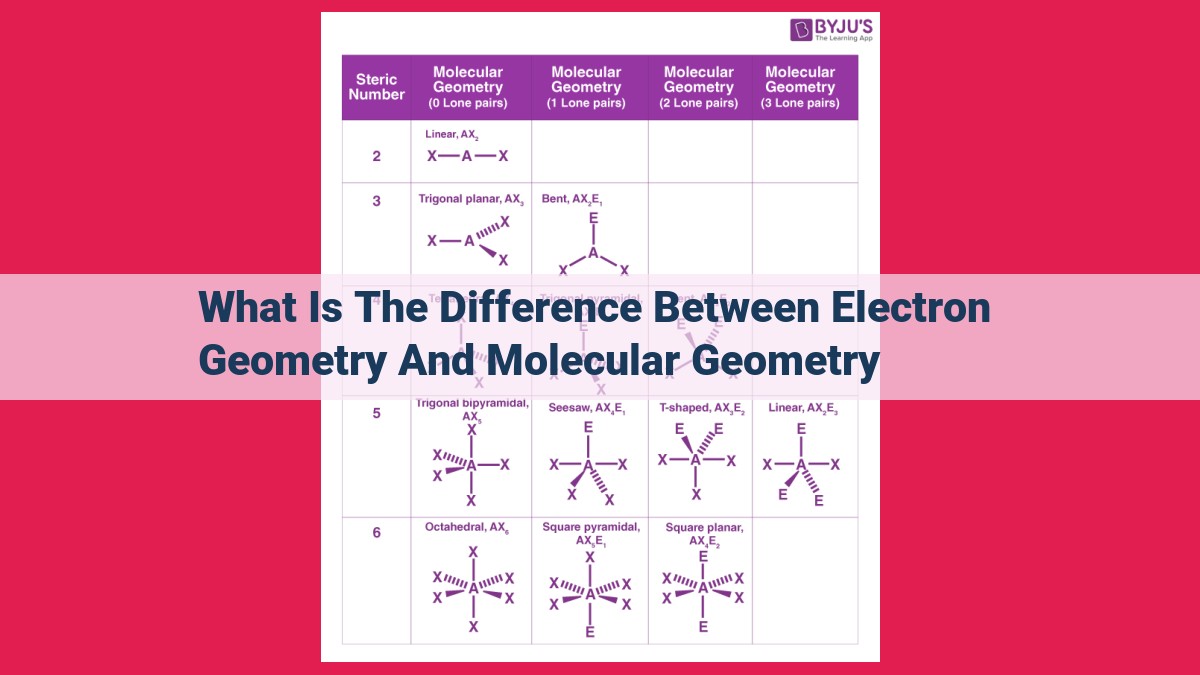
Electron geometry describes the spatial distribution of electrons around a central atom, influenced by factors like valence electrons, lone pairs, and hybridization. Molecular geometry, on the other hand, focuses on the overall shape of a molecule, determined by the arrangement of atoms and influenced by factors like valence electrons, lone pairs, hybridization, and electronegativity. The key differences lie in their focus, the electrons considered (valence vs. valence and core), the type of repulsion (electron-electron vs. electron-nucleus), and the specific influence of lone pairs and hybridization.
Electron and Molecular Geometry: Unraveling the Invisible Architecture of Molecules
In the realm of chemistry, molecules dance in an intricate ballet, their movements dictated by the unseen forces of electron and molecular geometry. Understanding these concepts is akin to deciphering the blueprint of the universe, unlocking secrets that govern the behavior and properties of matter.
Electron Geometry: The Dance of Electrons
Imagine electrons as tiny magnets, each carrying an invisible charge. The way these electrons arrange themselves around an atom forms a unique shape, known as electron geometry. It’s like a celestial waltz, where the electrons strive to minimize their repulsion, creating a symmetrical arrangement. Factors like the number of valence electrons, the presence of lone pairs, and hybridization influence this ethereal choreography.
Molecular Geometry: The Shape of Molecules
In contrast, molecular geometry describes the overall shape of a molecule. It’s determined not only by the electron geometry but also by the arrangement of atoms around a central atom. Lone pairs, with their space-hogging nature, can subtly alter the molecular shape, just as a wayward dancer can disrupt the rhythm of a group.
Distinguishing Electron and Molecular Geometry
While electron and molecular geometry are intricately intertwined, they are distinct concepts. Electron geometry focuses on the electron distribution around a single atom, while molecular geometry considers the arrangement of atoms within an entire molecule. Additionally, valence electrons play a central role in electron geometry, whereas both valence and core electrons contribute to molecular geometry.
Importance of Understanding Geometry
Deciphering these invisible geometries is not merely an academic pursuit. It holds immense practical significance. By predicting molecular shape, we can unravel the secrets behind molecular bonding, explain the physical and chemical properties of substances, design new materials with tailored properties, and even predict reactivity. It’s a key to unlocking the mysteries of the chemical world.
Electron Geometry: The Dance of Electrons Around Atoms
In the realm of chemistry, electron geometry plays a pivotal role in shaping the spatial arrangement of electrons around an atom. Think of electrons as tiny, negatively charged particles that orbit the positively charged nucleus at the heart of an atom. The distribution of these electrons governs the atom’s overall shape, determining its unique characteristics.
Electron geometry is closely intertwined with several fundamental concepts:
-
Valence electrons: Electrons occupying the outermost shell of an atom, which actively participate in chemical bonding.
-
Lone pairs: Pairs of electrons that are not involved in any chemical bonds and occupy their own distinct orbitals.
-
Hybridization: A process where atomic orbitals combine to form hybrid orbitals with different shapes and energies, influencing electron distribution.
-
Electronegativity: A measure of an atom’s ability to attract electrons in a chemical bond, affecting electron distribution and the shape of the electron cloud.
Understanding electron geometry is essential for comprehending the behavior of atoms and molecules, and for predicting their chemical properties. For instance, the arrangement of electrons around an atom dictates the type of chemical bonds it can form, its reactivity, and even its magnetic properties.
By deciphering the intricacies of electron geometry, chemists gain invaluable insights into the building blocks of matter and uncover the fascinating world of molecular interactions.
Molecular Geometry: Unveiling the Intriguing Shapes of Molecules
Understanding Molecular Geometry
Imagine a group of atoms, like a cluster of stars, orbiting around a central atom like a miniature solar system. The arrangement of these atoms determines the overall shape of the molecule. This intricate dance of atoms defines what we call molecular geometry.
Unlike electron geometry, which focuses solely on the distribution of electrons around an atom, molecular geometry encompasses the arrangement of entire atoms within a molecule. Valence electrons, lone pairs, and hybridization, play a crucial role in shaping molecular geometry.
Bonding and Molecular Shape
The number and arrangement of covalent bonds between atoms dictate the molecular shape. For instance, a molecule with four bonding pairs of electrons will adopt a tetrahedral shape, while a molecule with three bonding pairs will form a trigonal planar shape.
Lone Pairs and Molecular Geometry
Lone pairs of electrons, which are not involved in bonding, can also influence molecular geometry. They push away bonding electron pairs, causing the molecule to deviate from idealized shapes. For example, a molecule with two lone pairs on the central atom will adopt a bent shape instead of a tetrahedral shape.
Hybridization and Molecular Geometry
Hybridization, the mixing of atomic orbitals, plays a significant role in determining the shape of the molecule. Hybridized orbitals create a specific arrangement of electron pairs around the central atom, which in turn dictates the molecular geometry.
Applications of Molecular Geometry
The understanding of molecular geometry has revolutionized our understanding of matter. It enables scientists to:
- Predict the shape of molecules based on their structural formulas.
- Understand the nature of molecular bonding, including the strength and orientation of chemical bonds.
- Explain the physical and chemical properties of substances, such as their polarity, boiling point, and reactivity.
- Design new materials with tailored properties for specific applications.
- Predict the reactivity of molecules, aiding in the development of pharmaceuticals and catalysts.
Electron and Molecular Geometry: Key Differences
In the realm of chemistry, understanding the spatial arrangement of atoms and electrons is crucial. Electron geometry and molecular geometry, two distinct yet intertwined concepts, provide valuable insights into the behavior and properties of various substances.
Focus: Electron vs. Atomic Arrangement
- Electron geometry: Describes the arrangement of electrons around a central atom.
- Molecular geometry: Describes the arrangement of atoms around a central atom.
Valence Electrons
- Electron geometry: Considers only valence electrons.
- Molecular geometry: Considers both valence and core electrons.
Repulsion
- Electron geometry: Electrons repel each other, minimizing electron-electron repulsion.
- Molecular geometry: Electrons repel each other and repel the nucleus, determining the overall molecular shape.
Lone Pairs
- Electron geometry: Lone pairs (unbonded electrons) influence electron geometry by increasing electron density and creating more repulsive forces.
- Molecular geometry: Lone pairs do not affect molecular geometry, as they do not repel other atoms.
Hybridization
- Electron geometry: Hybridization determines the electron geometry by mixing atomic orbitals.
- Molecular geometry: Hybridization indirectly influences molecular geometry by altering the electron geometry.
Electronegativity
- Electron geometry: Electronegativity, the ability of an atom to attract electrons, influences electron distribution and electron geometry.
- Molecular geometry: Electronegativity also affects molecular geometry by influencing the distribution of charge and electron density around the molecule.
Applications of Electron and Molecular Geometry: Unraveling the Microscopic World
Electron and molecular geometry, often intertwined in intricacies, provide valuable insights into the microscopic realm that govern the behavior of molecules. Let’s delve into their captivating applications:
Unlocking Molecular Shapes: A Blueprint for Structure
Electron and molecular geometry determine molecular shapes, from the simplest to the most complex. By understanding the interplay between these geometries, scientists can predict the three-dimensional structures of molecules, unlocking essential information for drug design, protein folding, and materials science.
Illuminating Molecular Bonding: Deciphering the Forces that Bind
Molecular geometry reflects the underlying bonding patterns within molecules. It provides clues to the strength and nature of covalent, ionic, and metallic bonds, revealing the fundamental forces that hold molecules together. This knowledge empowers scientists to manipulate molecular interactions, designing substances with tailored properties.
Explaining Physical and Chemical Properties: Behavior Unveiled
Electron and molecular geometry influence the physical and chemical properties of substances. They explain melting points, boiling points, solubility, polarity, and reactivity. By understanding these relationships, researchers can design materials with specific thermal, electrical, and optical properties, leading to breakthroughs in fields such as energy storage, electronics, and catalysis.
Designing Novel Materials: Engineering Matter at the Nanoscale
Combining knowledge of electron and molecular geometry, scientists can engineer new materials with extraordinary properties. By optimizing the spatial arrangement of atoms and molecules, they can create materials with tailored strength, flexibility, conductivity, and functionality. These engineered materials pave the way for advanced technologies in fields such as biomedicine, electronics, and renewable energy.
Predictive Power: Unveiling Reactivity and Beyond
Electron and molecular geometry provide a framework for predicting the reactivity of molecules. They indicate the most reactive sites and facilitate the design of catalysts that accelerate chemical reactions. This in-depth understanding empowers chemists to synthesize new molecules, develop more efficient processes, and explore novel applications in pharmaceuticals, energy, and environmental science.