Understand Chemical Bonding With Electron Dot Diagrams (Lewis Structures)
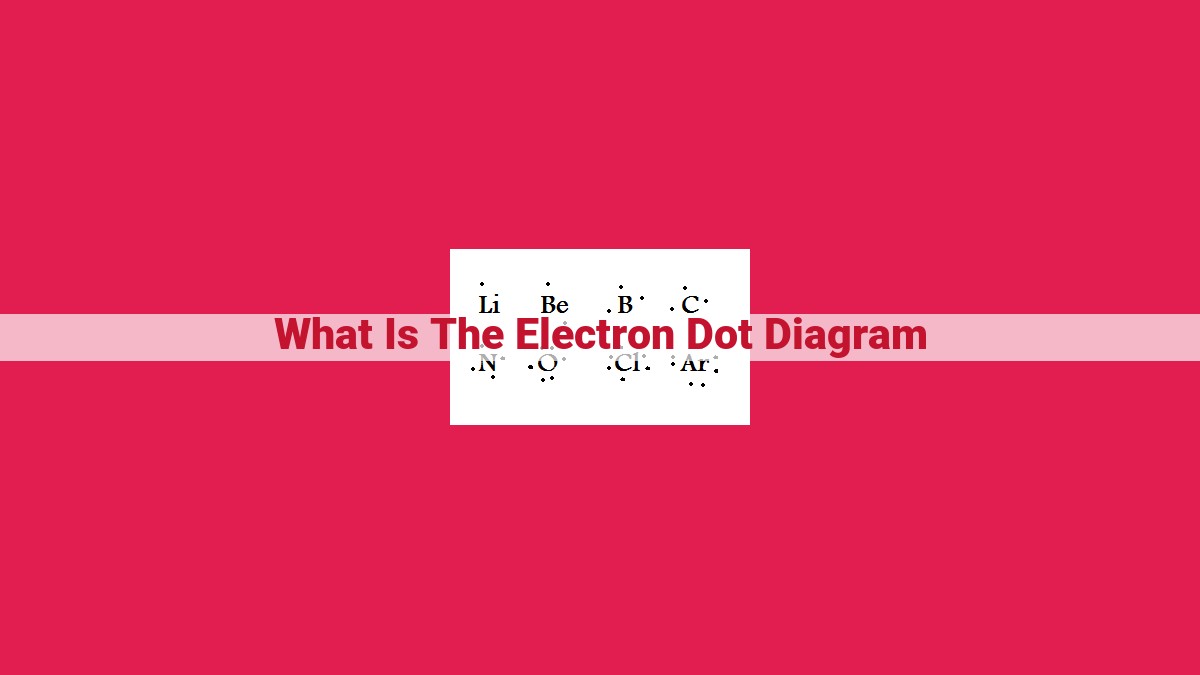
Electron dot diagrams (Lewis structures) visually depict the valence electrons of atoms, forming the foundation for understanding chemical bonding and molecular geometry. These diagrams represent valence electrons as dots around the atomic symbol, with each dot representing a single electron. Electron pairs are key elements in electron dot diagrams, as they guide the formation of covalent bonds, which result from the sharing of electron pairs between atoms. The octet rule, a crucial principle in electron dot diagrams, states that atoms generally strive to have eight valence electrons to achieve stability.
- Define and explain the purpose of electron dot diagrams (Lewis structures).
Electron Dot Diagrams: Unlocking the Secrets of Chemical Bonding
What if you could visualize the intricate dance of electrons within molecules? Electron dot diagrams, also known as Lewis structures, offer a simple yet powerful tool to do just that. Imagine each electron as a tiny planet, orbiting the nucleus of an atom. In these diagrams, electrons are represented by dots placed around the atomic symbol.
Electrons: The Building Blocks of Matter
At the heart of electron dot diagrams lies the concept of valence electrons. These are the electrons that roam freely outside the atom’s core, eager to interact with other atoms. The number of valence electrons determines an atom’s chemical behavior and the types of bonds it can form.
Electron Pairs: The Key to Bonding
Electrons love to play in pairs, and they do so by forming covalent bonds. In electron dot diagrams, these pairs are represented by lines connecting the atomic symbols. When atoms share valence electrons, they become interconnected, forming molecules.
The Octet Rule: A Guiding Principle
The octet rule is a guiding principle in electron dot diagram formation. It states that atoms tend to form bonds until they have a complete set of eight valence electrons. This stable configuration ensures that atoms achieve a balanced and energetically favorable state.
Covalent Bonds: Sharing the Electron Wealth
Covalent bonds are formed when atoms share valence electrons, resulting in the formation of a shared electron cloud. Electron dot diagrams vividly illustrate this sharing phenomenon, creating a picture of the interconnectedness of atoms within a molecule.
Valence Electrons: The Building Blocks of Electron Dot Diagrams
In the realm of chemistry, understanding the behavior of electrons is paramount. Electron dot diagrams, also known as Lewis structures, are valuable tools that provide a visual representation of electron arrangements and bonding in molecules. At the heart of these diagrams lie valence electrons, the electrons that play a crucial role in determining a molecule’s chemical properties.
Valence electrons reside in the outermost energy level of an atom, yearning to interact with other atoms to achieve stability. The number of valence electrons an atom possesses is determined by its position on the periodic table. For instance, group 1 elements like sodium have a solitary valence electron, while group 16 elements such as oxygen boast a generous eight valence electrons.
The relationship between valence electrons and electron pairs is fundamental. Electron pairs are pairs of valence electrons that share a bond, the glue that holds atoms together. By examining the number of valence electrons an atom has, we can predict the number of electron pairs it can form. For example, carbon, with four valence electrons, can form four electron pairs, potentially connecting to four other atoms.
Understanding valence electrons and electron pairs is vital for constructing accurate electron dot diagrams. These diagrams provide a roadmap of a molecule’s electronic structure, helping us visualize the formation of covalent and ionic bonds, predict molecular geometry, and comprehend the reactivity of chemical substances.
Electron Pairs: The Building Blocks of Electron Dot Diagrams
In the realm of chemistry, where atoms dance and bonds form, electron dot diagrams (Lewis structures) serve as visual representations of molecular architecture. At the heart of these diagrams lie electron pairs, the fundamental building blocks that determine the shape and behavior of molecules.
Electron pairs are formed when atoms share their valence electrons, those outermost electrons that participate in chemical reactions. Each electron pair is represented by a pair of dots placed around the atomic symbol of the corresponding element. For instance, in the electron dot diagram of chlorine (Cl), seven dots encircle the Cl symbol, signifying its seven valence electrons.
The arrangement of electron pairs around atoms is governed by the octet rule, a guiding principle that states that atoms tend to stabilize by acquiring eight valence electrons. This explains why atoms often form electron pairs with other atoms, sharing electrons to achieve a complete octet of valence electrons. The resulting Lewis dot structure represents the electron configuration of the molecule, providing insights into its chemical properties.
For example, consider the electron dot diagram of carbon dioxide (CO2). Carbon has four valence electrons, while each oxygen atom has six. To attain an octet, carbon shares two of its valence electrons with each oxygen atom, forming two covalent bonds. The resulting Lewis dot structure reveals the linear shape of CO2, with the carbon atom at the center and the two oxygen atoms attached on either side.
Thus, electron pairs play a crucial role in constructing electron dot diagrams. They represent the shared electrons between atoms and serve as the foundation for understanding the formation and properties of molecules.
The Octet Rule: Guiding the Formation of Electron Dot Diagrams
The octet rule is a cornerstone of chemistry, guiding the formation and behavior of molecules. It states that atoms tend to gain, lose, or share electrons to achieve a stable configuration with eight valence electrons in their outermost energy level—a full octet.
Valence Electrons and the Octet Rule
Valence electrons are the electrons in an atom’s outermost energy level, and they determine the atom’s chemical properties. The octet rule governs the arrangement of these electrons. Atoms are most stable when their valence electrons are arranged in a complete octet, which resembles the electron configuration of noble gases.
Electron Pairs and the Octet Rule
Electron pairs are pairs of electrons that occupy the same energy level. In electron dot diagrams, these pairs are represented by dots placed around the atom’s symbol. The octet rule dictates that atoms strive to have four electron pairs in their valence level—two lone pairs (two electrons paired with each other) or four shared pairs (covalent bonds with other atoms).
Lewis Dot Structures and the Octet Rule
Lewis dot structures are diagrams that represent the arrangement of valence electrons in a molecule. By following the octet rule, chemists can create Lewis dot structures that predict the molecular geometry and chemical properties. Deviations from the octet rule occur when atoms have an odd number of valence electrons or when the molecule forms resonance structures.
Understanding the octet rule is essential for comprehending the behavior of atoms and molecules. It provides a framework for predicting electron configurations, molecular shapes, and chemical bonding, making it a crucial concept in the study of chemistry.
Covalent Bonds: The Bonds Formed by Electron Dot Diagrams
Covalent bonds are the backbone of molecular chemistry, holding atoms together to form a vast array of compounds. These bonds arise when valence electrons, the outermost electrons in an atom, are shared between two or more atoms.
Electron dot diagrams, also known as Lewis structures, provide an insightful visual representation of these covalent bonds. They depict each atom as a symbol surrounded by dots, which symbolize valence electrons. When atoms share valence electrons, these dots are paired together to form electron pairs.
In electron dot diagrams, covalent bonds are depicted as lines connecting the shared electron pairs between atoms. These lines signify that the valence electrons are shared equally, creating a covalent bond. The number of covalent bonds formed depends on the number of valence electrons available for sharing.
Molecular Geometry and Covalent Bonds
The arrangement of covalent bonds determines the molecular geometry. This three-dimensional shape of a molecule affects its properties, including chemical reactivity and physical characteristics.
When two atoms share one covalent bond, the molecule adopts a linear geometry. Three covalent bonds result in a trigonal planar geometry, and four covalent bonds give rise to a tetrahedral geometry. As the number of covalent bonds increases, the shape of the molecule becomes more complex.
Understanding covalent bonds through electron dot diagrams is essential for comprehending the formation and behavior of molecules. These diagrams provide a visual framework that allows chemists to predict molecular structure, explain chemical reactivity, and design new materials.
Ionic Bonds: Electron Transfer and Dot Diagrams
Ionic bonds are a type of chemical bond that forms when one atom transfers one or more electrons to another atom. This transfer of electrons creates two oppositely charged ions: a positively charged cation and a negatively charged anion. Electron dot diagrams, also known as Lewis structures, can be used to represent the electron transfer and resulting ionic bond formation.
In an ionic bond, the atom that loses electrons becomes the cation. The number of electrons lost is equal to the number of positive charges on the cation. The atom that gains electrons becomes the anion. The number of electrons gained is equal to the number of negative charges on the anion.
Electron dot diagrams show the valence electrons of each atom involved in the ionic bond. Valence electrons are the electrons in the outermost energy level of an atom. In an ionic bond, the valence electrons of the metal atom are transferred to the nonmetal atom.
The formation of an ionic bond can be illustrated using electron dot diagrams. For example, consider the formation of sodium chloride (NaCl). Sodium (Na) has one valence electron, while chlorine (Cl) has seven valence electrons. To achieve a stable electron configuration, sodium transfers its one valence electron to chlorine. This results in the formation of a sodium ion (Na+) and a chloride ion (Cl-).
The electron dot diagram for sodium chloride shows the transfer of the valence electron from sodium to chlorine:
Na: . + :. Cl
The resulting ionic bond is strong because the oppositely charged ions are attracted to each other. Ionic bonds are commonly found in compounds formed between metals and nonmetals.
Molecular Geometry: The Shapes Determined by Electron Dot Diagrams
Electron dot diagrams not only reveal bonding arrangements but also the molecular geometry of the resulting compound. The shape of a molecule directly relates to its bonding pattern and affects its chemical properties and behavior.
Covalent Bonds and Molecular Geometry:
When atoms share electron pairs to form covalent bonds, the resulting arrangement of these electron pairs determines the molecular geometry. The most common electron pair arrangements are:
- Linear: Two atoms bonded by a single covalent bond, with electron pairs aligned linearly.
- Trigonal Planar: Three atoms bonded to a central atom by single covalent bonds, forming a flat triangular shape.
- Tetrahedral: Four atoms bonded to a central atom by single covalent bonds, forming a tetrahedral shape.
Ionic Bonds and Molecular Geometry:
In ionic bonds, electrons are transferred from one atom to another. The resulting ions have opposite charges, attracting each other to form an ionic bond. The arrangement of these ions determines the molecular geometry of the ionic compound.
Common ionic geometries include:
- Face-Centered Cubic: Ions arranged in a cubic lattice with ions occupying the centers of each face.
- Body-Centered Cubic: Ions arranged in a cubic lattice with an ion at the center of the cube.
- Hexagonal Close-Packed: Ions arranged in a hexagonal lattice with ions occupying the centers of each hexagon.
Relationship Between Electron Dot Diagrams and Molecular Geometry:
Electron dot diagrams provide a visual representation of the electron pairs involved in bonding. By observing the electron pair arrangement on the diagram, we can predict the molecular geometry of the compound. For instance:
- A molecule with a linear electron dot diagram will have a linear molecular geometry.
- A molecule with a trigonal planar electron dot diagram will have a trigonal planar molecular geometry.
Understanding molecular geometry is crucial in chemistry as it influences a molecule’s properties, such as reactivity, magnetism, and solubility. By analyzing electron dot diagrams, we gain valuable insights into the structure and properties of chemical compounds.