Electron Configuration Of Sodium (Na): Understanding Its Properties And Position In The Periodic Table
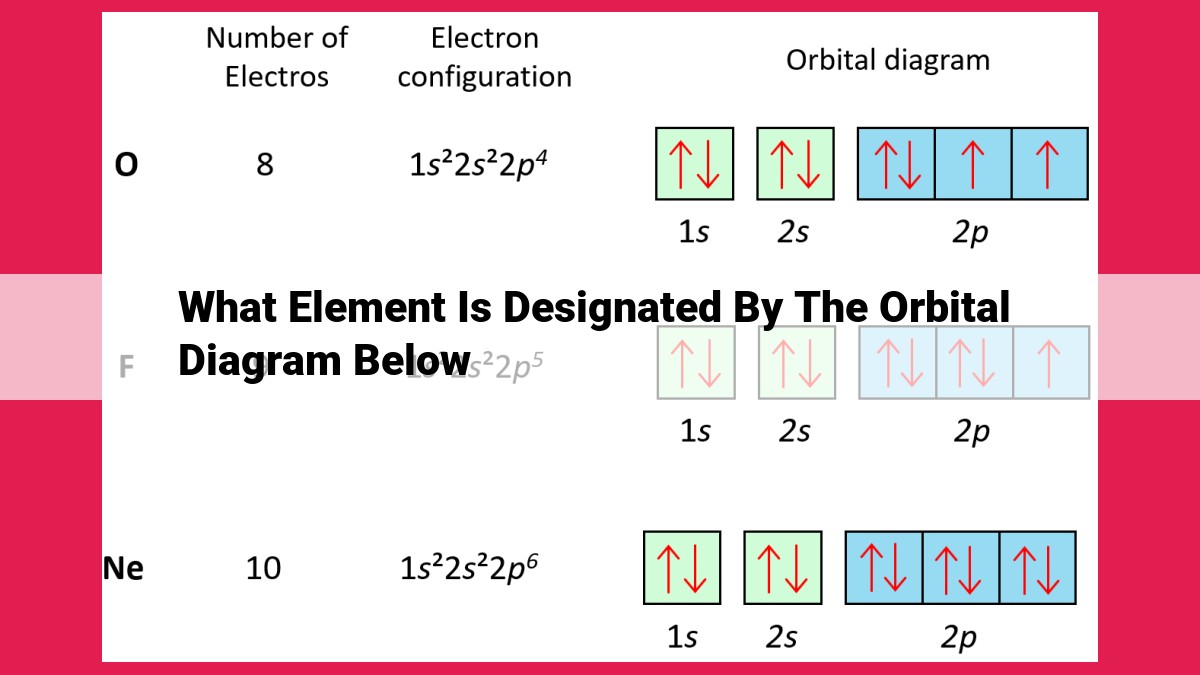
The orbital diagram provided corresponds to an element with the following electron configuration: 1s² 2s² 2p⁶ 3s¹ This configuration matches that of the element Sodium (Na), which belongs to Group 1 (Alkali Metals) and Period 3 of the Periodic Table.
Unraveling the Secrets of Atomic Number: A Key to the Periodic Puzzle
In the realm of chemistry, the Periodic Table is an indispensable tool. It organizes elements based on their fundamental properties and provides valuable insights into their behavior. One of the critical pieces of information embedded within the Periodic Table is the Atomic Number. Understanding this concept is like unlocking a secret code that unlocks the mysteries of the atomic world.
Simply put, Atomic Number refers to the number of protons residing in the nucleus of an atom. Protons are tiny, positively charged particles that define the identity of an element. Each element has a unique Atomic Number, like a fingerprint, making it distinct from all others. The Atomic Number is often denoted by the symbol Z.
For instance, let’s take Hydrogen, the first element in the Periodic Table. Hydrogen has an Atomic Number of 1, indicating that every Hydrogen atom has exactly one proton in its nucleus. Carbon, on the other hand, has an Atomic Number of 6, meaning it has six protons in each atom.
The Atomic Number plays a vital role in determining the Proton Number and Element Symbol. For any given element, the Atomic Number and Proton Number are identical. The element is commonly represented by a one or two-letter symbol, which is derived from its Latin name. For example, Hydrogen is abbreviated as H and Carbon as C.
Period: Unveiling the Horizontal Rows of the Periodic Table
In the world of chemistry, the Periodic Table stands as a comprehensive guide to the elements that make up our universe. Within this structured matrix, horizontal rows known as periods play a crucial role in understanding the properties and behavior of elements.
Conceptually, each period represents a specific energy level or electron shell. Electrons occupy these shells in a hierarchical manner, with the lowest energy levels being filled first. As you move across a period from left to right, the atomic number of the elements increases, indicating an increase in the number of electrons. Consequently, the elements within the same period share a common energy level, affecting their chemical properties and reactivity.
For instance, elements in the first period, such as hydrogen and helium, possess a single electron shell with a maximum capacity of two electrons. In contrast, elements in the second period, like carbon and oxygen, have two energy levels or electron shells, with a combined capacity of eight electrons. This variation in the number of electron shells accounts for the diverse chemical properties observed across the periods.
Groups: Vertical Columns, Valence Electrons, and Chemical Properties
Imagine the Periodic Table as a grid, with elements arranged in neat rows and columns. These columns are known as groups, and they play a crucial role in understanding an element’s chemical behavior.
Each group represents elements with similar valence electrons—the electrons in their outermost energy level. These valence electrons are primarily responsible for chemical reactions. For instance, elements in Group 1 (the alkali metals) have a single valence electron, making them highly reactive and eager to form bonds with other elements.
The number of valence electrons also determines the chemical properties of an element. Elements in the same group share similar chemical properties because they have the same number of valence electrons. Take halogens in Group 17: they all have seven valence electrons, giving them a strong tendency to acquire an electron and form stable bonds.
Understanding groups can help us predict the reactivity and bonding behavior of elements. By grouping elements with similar valence electrons, the Periodic Table provides a systematic way to organize and understand the vast array of elements in the universe.
Orbital Shape and Energy: Unraveling the Three-Dimensional Quantum World
The Periodic Table, an indispensable tool for chemists, physicists, and students alike, organizes elements based on their properties and behaviors. To grasp the intricate workings of the Periodic Table, it’s essential to understand orbital shape and energy. This article will delve into these concepts, unveiling the fascinating world of electron orbitals.
Orbital Shape: A Quantum Jigsaw Puzzle
Electron orbitals, denoted by different shapes like spheres, dumbbells, and cloverleaves, are three-dimensional regions where electrons are most likely to be found. These shapes are governed by a complex set of mathematical equations and quantum numbers, which describe the energy, angular momentum, and spin of electrons.
Subshells and Quantum Numbers
Orbitals can further be classified into subshells, labeled as s, p, d, and f. Each subshell corresponds to a specific energy level and has a different number of orbital shapes. The arrangement of electrons within these subshells is known as electron configuration.
Energy Levels: A Hierarchical Dance
Orbitals within a subshell exist at different energy levels, with s being the lowest and f being the highest. This energy hierarchy influences the chemical properties of elements and their position in the Periodic Table.
Shells and Periods
Orbitals are grouped into shells, which correspond to periods on the Periodic Table. Each shell has a specific number of electrons, and the number of shells determines the period the element belongs to.
Groups and Valence Electrons
Electrons in the outermost shell are called valence electrons, which play a crucial role in determining the chemical properties of elements. Elements in the same group have the same number of valence electrons and therefore share similar chemical behaviors.
Understanding orbital shape and energy is a gateway to comprehending the intricate world of chemistry and the periodic relationships between elements. By unraveling these fundamental concepts, we gain a deeper appreciation for the organization and behavior of matter in the universe.