Electron Carrier Molecules: The Unsung Heroes Of Life’s Cellular Processes
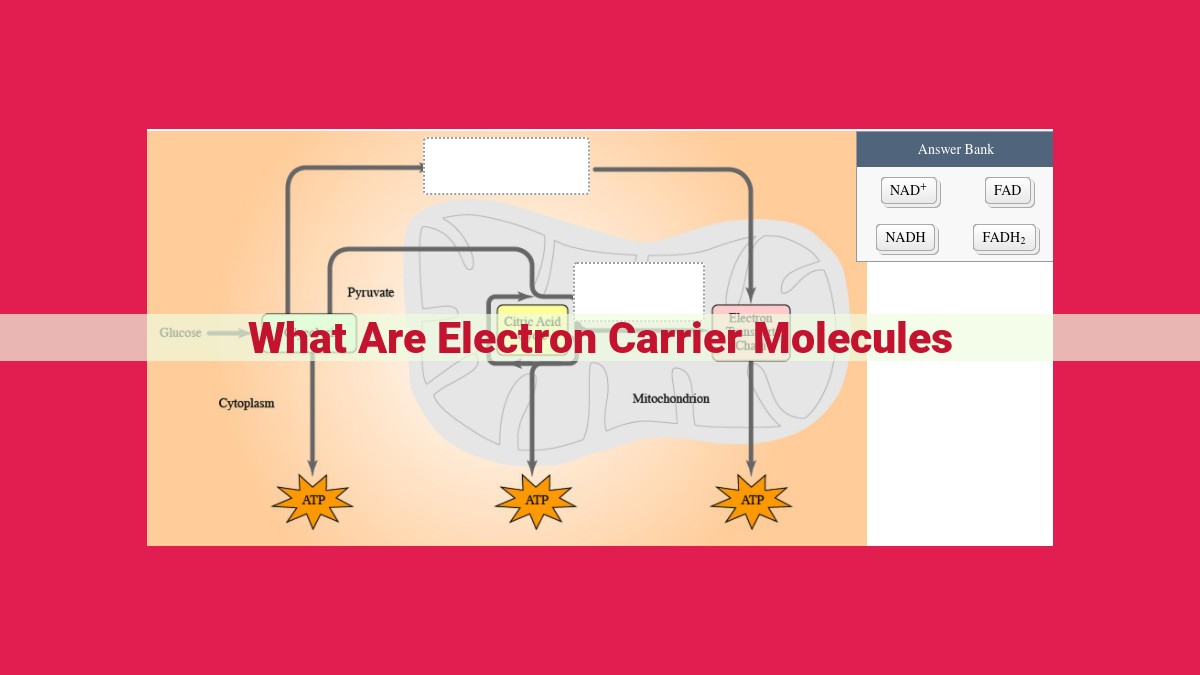
Electron carrier molecules are vital in biochemical reactions, facilitating redox electron transfers. They accept electrons from donor molecules and transfer them to acceptor molecules. Coenzymes, such as FMN, FAD, NAD+, NADP+, ubiquinone, and cytochromes, act as electron carriers in various processes like oxidative phosphorylation, glycolysis, and the citric acid cycle. Their efficient electron transfer enables energy production, metabolism, and other cellular functions, highlighting their indispensable role in sustaining life.
Electron Carrier Molecules: The Unsung Heroes of Life
In the intricate tapestry of life, biochemical reactions play a pivotal role, driving the myriad processes that sustain our existence. At the heart of these reactions lie electron carrier molecules, the unsung heroes that orchestrate the flow of electrons, enabling life’s essential functions.
What are Electron Carrier Molecules?
Electron carrier molecules are specialized molecules that possess the remarkable ability to transfer electrons from one molecule to another. This property makes them indispensable in a vast array of biochemical reactions, from the respiration that fuels our cells to the synthesis of countless biomolecules.
The Importance of Electron Transfer
Electron transfer is a fundamental process in living organisms. It drives redox reactions, chemical reactions that involve the transfer of electrons between reactants. These reactions are crucial in energy metabolism, the production of biological molecules, and the detoxification of harmful substances.
Coenzymes as Electron Carriers
Many electron carrier molecules are known as coenzymes, which act as essential partners to enzymes, the workhorses of biochemical reactions. Notable examples of coenzymes include:
- Flavin Mononucleotide (FMN) and Flavin Adenine Dinucleotide (FAD): These coenzymes participate in a wide range of redox reactions, including those involved in oxidative phosphorylation and the citric acid cycle.
- Nicotinamide Adenine Dinucleotide (NAD+) and Nicotinamide Adenine Dinucleotide Phosphate (NADP+): These coenzymes play vital roles in energy metabolism, acting as electron carriers in glycolysis and oxidative phosphorylation, respectively.
Redox Reactions and Electron Transfer: The Dance of Molecules
In the intricate world of biochemistry, electron carrier molecules play a pivotal role in the dance of chemical reactions. They are the couriers that transport electrons from one molecule to another, facilitating a continuous flow of energy that sustains life.
Redox reactions are the heart of this electron dance. They involve the exchange of electrons between molecules, where one molecule is oxidized (loses electrons) while another is reduced (gains electrons). Electron carrier molecules act as the bridge between these reactions, mediating the transfer of electrons.
Imagine a relay race, where electron carrier molecules are the runners. They receive electrons from one molecule and swiftly pass them on to the next. This continuous relay allows electrons to travel through a series of reactions, releasing energy that drives cellular processes.
The Role of Coenzymes in Electron Transfer: Facilitating Life’s Vital Reactions
In the intricate tapestry of life, biochemical reactions play an indispensable role. Among these reactions, electron transfer stands out as a fundamental process that drives numerous physiological processes. Coenzymes, the unsung heroes of electron transfer, serve as vital intermediaries, ensuring the seamless flow of electrons that sustains life.
As electron carriers, coenzymes act as middlemen, transporting electrons between enzymes and their substrates. This intricate dance of electron exchange underpins a multitude of biochemical pathways, ranging from energy production to nerve impulse transmission.
Specific coenzymes play distinct roles in these electron-shuffling processes. Flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD), for instance, participate in oxidative phosphorylation and the citric acid cycle, two energy-generating pathways crucial for cellular function.
Nicotinamide adenine dinucleotide (NAD+) and nicotinamide adenine dinucleotide phosphate (NADP+) take center stage in energy metabolism. NAD+ acts as a primary electron carrier in glycolysis, while NADP+ plays a pivotal role in oxidative phosphorylation, the powerhouse of the cell.
The intricate electron transfer choreography extends beyond these coenzymes. Ubiquinone and cytochromes, embedded within the mitochondrial electron transport chain, form a relay system that efficiently transports electrons, generating the energy that fuels cellular activities.
In conclusion, coenzymes are the unsung heroes of electron transfer, the invisible force behind life’s most essential biochemical reactions. Their ability to facilitate the seamless flow of electrons underscores their profound significance in maintaining the delicate balance of life.
Flavin Mononucleotide (FMN) and Flavin Adenine Dinucleotide (FAD)
- Discuss the involvement of FMN and FAD in redox reactions, emphasizing their role in oxidative phosphorylation and the citric acid cycle.
Flavin Mononucleotide (FMN) and Flavin Adenine Dinucleotide (FAD): Essential Electron Carriers in Cellular Metabolism
In the intricate symphony of biochemical reactions that sustain life, electron carrier molecules play a pivotal role. Among these molecules, Flavin Mononucleotide (FMN) and Flavin Adenine Dinucleotide (FAD) stand out as essential mediators of energy metabolism, particularly in oxidative phosphorylation and the citric acid cycle.
FMN and FAD are derivatives of vitamin B2 (riboflavin). They participate in redox reactions, where electrons are transferred between molecules. In oxidative phosphorylation, which occurs in the mitochondria, FMN and FAD serve as electron acceptors in the electron transport chain. Electrons are passed from one carrier to another, ultimately to oxygen, generating energy in the form of ATP.
In the citric acid cycle, FMN and FAD play a crucial role in generating NADH and FADH2, two high-energy electron carriers that carry electrons to the electron transport chain. FMN is involved in the succinate dehydrogenase reaction, while FAD is a cofactor in the isocitrate dehydrogenase and malate dehydrogenase reactions.
**The vital contributions of FMN and FAD in cellular metabolism highlight the importance of electron carrier molecules in sustaining life. Without these essential mediators, energy production would be severely impaired, leading to a disruption of cellular function and ultimately the health of the organism.**
The Dynamic Duo: NAD+ and NADP+ in Energy Metabolism
In the bustling world of cellular energy, two essential players take center stage: Nicotinamide Adenine Dinucleotide (NAD+) and its close cousin, Nicotinamide Adenine Dinucleotide Phosphate (NADP+). These dynamic molecules serve as electron carriers, orchestrating the intricate dance of energy production that powers every living cell.
NAD+ and Glycolysis: Fueling the Cell
NAD+ stands as a crucial partner in glycolysis, the first stage of cellular respiration. As glucose molecules break down, NAD+ deftly captures electrons, transforming into NADH and H+ ions. These electron-laden molecules then embark on a journey to the mitochondria, carrying their energetic cargo.
NADP+ and Oxidative Phosphorylation: The Powerhouse of the Cell
NADP+ plays an equally vital role in the mitochondria, supporting the electron transport chain. This chain of protein complexes serves as an energetic conveyor belt, shuttling electrons from NADH and FADH2 to create an electrochemical gradient. The energy harnessed from this gradient is used to synthesize ATP, the universal energy currency of cells.
The Significance of NAD+/NADP+ Balance
Maintaining a healthy balance of NAD+ and NADP+ is paramount for cellular well-being. These molecules serve as signaling molecules, influencing gene expression and metabolic pathways. NAD+ levels, in particular, have been linked to aging, sirtuins (longevity proteins), and cellular stress response.
NAD+ and NADP+ are indispensable electron carriers, playing a pivotal role in energy metabolism. Their contributions to glycolysis and oxidative phosphorylation make them essential for cellular energy production. Moreover, their signaling functions further underscore their importance in maintaining overall cellular health. These dynamic molecules embody the intricate and interconnected nature of life, highlighting the vital importance of electron transfer in the ceaseless quest for energy.
Ubiquinone and Cytochromes: Guardians of the Mitochondrial Electron Dance
At the heart of our cells lies a mitochondria, a tiny powerhouse that generates energy. Within these powerhouses, ubiquinone and cytochromes play a pivotal role in a danse macabre of electrons, driving energy production and sustaining life.
Ubiquinone: The Electron Shuttle
Ubiquinone (coenzyme Q) is a mobile electron carrier embedded in the mitochondrial membrane. It shuttles electrons between the first two complexes of the electron transport chain (ETC).
As electrons flow in, ubiquinone undergoes a chemical dance, its molecular structure alternating between oxidized (ubiquinone) and reduced (ubiquinol) forms. This electron tango powers the pumping of protons across the mitochondrial membrane, creating an electrochemical gradient that ultimately generates ATP, the energy currency of the cell.
Cytochromes: The Orchestral Ensemble
Cytochromes are a suite of proteins that orchestrate the electron transfer process within the ETC. Each cytochrome contains a heme group, a porphyrin ring complexed with an iron ion, which allows them to undergo reversible redox reactions, accepting and donating electrons.
The cytochromes are arranged in a precise electron cascade, with each cytochrome passing electrons to the next in line. This orchestrated dance creates a thermodynamic gradient, driving the flow of electrons and pumping protons, further contributing to ATP production.
A Synergistic Symphony
The interplay between ubiquinone and cytochromes in the mitochondrial electron transport chain is a symphony of energy generation. Ubiquinone shuttles electrons, while cytochromes orchestrate the transfer, creating an electrochemical gradient that powers ATP synthesis.
This electron dance is the heartbeat of cellular respiration, providing the energy that fuels every aspect of our lives. Ubiquinone and cytochromes are the unsung heroes, diligently performing their roles in this vital process, ensuring that we breathe, move, and thrive.