Discover The Electron Configuration Of Helium: Unveiling Its Inert Nature
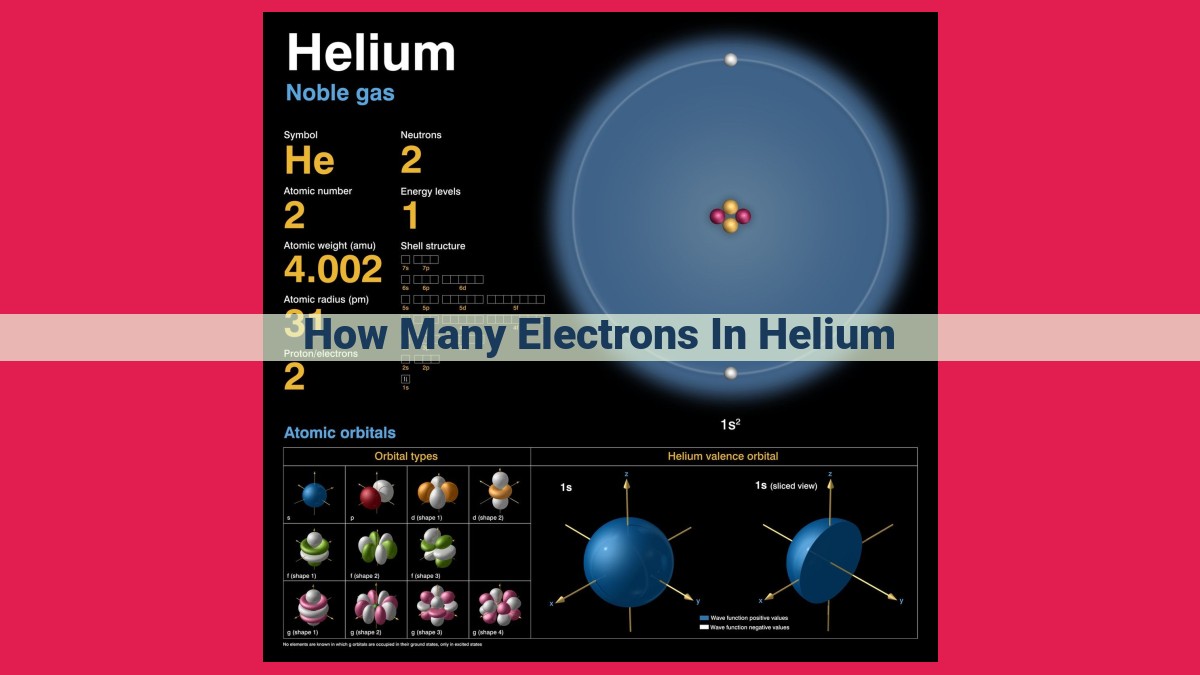
Helium, an element known for its inert nature, possesses unique properties determined by its electron configuration. With an atomic number of 2, helium occupies the second position in the Periodic Table. Understanding its electron configuration is crucial as it influences the number of electrons the element contains. Helium’s electron configuration, denoted as 1s², reveals that it has two electrons occupying the first energy level in the 1s orbital. This arrangement adheres to the Pauli Exclusion Principle, which states that no two electrons within an atom can have the exact same set of quantum numbers. Thus, helium possesses two electrons in its ground state, contributing to its stability and non-reactivity.
Helium: The Gas of Many Wonders, Unveiling Its Electron Configuration Mysteries
In the vast expanse of the universe, one element stands out for its exceptional lightness and unique properties: helium. This remarkable gas, with its ability to defy gravity, has captivated scientists and has found countless applications in fields ranging from aerospace to medicine. To unravel the secrets behind its extraordinary behavior, we delve into the heart of helium’s atomic structure, exploring the significance of its electron configuration.
Defining Helium and Its Enigmatic Properties
Helium, with the atomic symbol He, is the second element on the Periodic Table. Its atomic number, which we’ll delve into shortly, is 2. This number indicates the number of protons in its nucleus, which is the heart of the atom. Helium is renowned for its exceptional lightness, as it is the second lightest element after hydrogen. It is colorless, odorless, and tasteless, making it virtually undetectable by our senses.
Helium’s low density and lack of reactivity have made it a crucial component in various applications. It is used to fill balloons and party inflatables, providing that buoyant lift that makes them float. In the medical field, it is used in MRI scans, providing vivid images of the body’s internal structures. The inert nature of helium has also made it essential for welding and manufacturing processes, where it prevents unwanted chemical reactions.
Unraveling the Importance of Electron Configuration
To fully comprehend helium’s behavior, we must venture into the realm of electron configuration. This concept describes the arrangement of electrons around the atomic nucleus. Electrons, with their negative charge, occupy specific energy levels or orbitals, which are determined by their distance from the nucleus. The electron configuration of an element dictates many of its chemical and physical properties.
By understanding helium’s electron configuration, we can better grasp why it behaves the way it does. This knowledge enables us to predict its reactivity, its bonding capabilities, and its overall behavior in different chemical environments.
Helium’s Atomic Number: A Key to Understanding Its Electron Configuration
Understanding the intricate world of atoms and their properties is crucial for unraveling the secrets of our physical surroundings. One such building block of matter, helium, possesses fascinating characteristics that make it an indispensable element in scientific exploration.
The Concept of Atomic Number
At the heart of every atom lies a central core, the nucleus, where protons and neutrons reside. The number of protons within this nucleus defines the atomic number of an element, acting as a unique identification number in the periodic table. It’s like the passport of an element, telling us who it is and where it belongs.
Helium’s Position on the Periodic Table
Delving into the world of the periodic table, we find helium situated in the second group, the first period, with an atomic number of 2. This placement reveals that helium has two protons in its nucleus. Imagine this as a tiny, positively charged duo at the atom’s core.
Electron Configuration Concept:
- Define electron configuration and explain its significance.
- Introduce the idea of orbitals and how they relate to electron configuration.
Understanding Helium’s Electron Configuration
Helium, the second element on the Periodic Table, holds a special significance in the world of chemistry. Its unique properties have led to its widespread use in various applications. However, to fully grasp helium’s behavior, we must delve into the realm of electron configuration, a fundamental concept that unveils the arrangement of electrons within an atom.
Electron Configuration: A Guiding Force
Electron configuration is the distribution of electrons in different energy levels or orbitals around the atom’s nucleus. It plays a pivotal role in determining an element’s chemical properties. Each orbital, denoted by a letter (s, p, d, f), can accommodate a specific number of electrons. The 1s orbital, the lowest energy orbital, holds a maximum of two electrons.
Helium’s Electron Configuration: A Duet of Electrons
Helium’s electron configuration is represented as 1s². This means that helium has two electrons residing in its 1s orbital. This arrangement is stable and energetically favorable, making helium a noble gas. Noble gases are known for their low reactivity due to their complete electron shells, with helium being the lightest and simplest among them.
Pauli Exclusion Principle: The Enforcer of Quantum Laws
The Pauli Exclusion Principle governs the behavior of electrons within an atom. It dictates that no two electrons in the same atom can share the exact same quantum state. In other words, they cannot have the same set of four quantum numbers (n, l, ml, ms). This principle ensures that electrons occupy different energy levels or orbitals with distinct quantum numbers.
Helium’s unique electron configuration of 1s² is a defining characteristic that underlies its chemical inertness and its status as a noble gas. Understanding electron configuration is crucial for comprehending not only helium’s behavior but also the properties of all elements. By deciphering the electron configurations of atoms, scientists gain valuable insights into the fascinating world of chemistry and the interactions that shape the universe we live in.
Helium’s Electron Configuration: A Tale of Two Electrons
Prepare yourself to uncover the secrets of helium’s peculiar electron configuration! Helium, a fascinating element, boasts unique properties that make it essential for understanding the building blocks of our universe. In this blog post, we’ll embark on a journey to comprehend the significance of its electron arrangement, a crucial factor in determining its behavior and characteristics.
Atomic Number and Helium:
Each element in the periodic table is distinguished by its atomic number, which defines the number of protons in its nucleus. Helium, the second element, proudly holds an atomic number of 2, indicating two protons within its atomic core. This atomic number plays a pivotal role in identifying and categorizing elements.
Electron Configuration Concept:
Electron configuration represents the arrangement of electrons within an element’s energy levels or orbitals. These energy levels are like energy shells around an atom’s nucleus, holding electrons at specific distances. Understanding electron configuration is crucial for comprehending an element’s chemical properties and behavior.
Helium’s Electron Configuration:
Unveiling helium’s electron configuration, we discover a simple yet fundamental arrangement: 1s². This notation indicates that helium has two electrons residing in its 1s orbital. The 1s orbital represents the lowest energy level closest to the nucleus.
Arrangement of Electrons within the 1s Orbital:
A single 1s orbital can accommodate a maximum of two electrons. These two electrons reside within the 1s orbital with opposite spins. The concept of electron spin, a fundamental property of electrons, describes their intrinsic angular momentum. The Pauli Exclusion Principle governs this arrangement, ensuring that no two electrons within an atom can have the same set of quantum numbers, including spin.
In summary, helium’s electron configuration, 1s², signifies two electrons occupying its lowest energy level, the 1s orbital. This configuration is in accordance with the Pauli Exclusion Principle, which prevents electrons from sharing the same quantum state. Understanding helium’s electron arrangement provides valuable insights into its chemical behavior and its role in various applications, from cryogenics to lighter-than-air vehicles.
Helium’s Ground State: Unveiling the Electron Dance
In the tapestry of elements, helium stands apart with its enigmatic properties. Yet, understanding the dance of electrons within its atoms holds the key to unlocking its secrets.
Ground State: The Electron’s Haven
Every atom resides in a preferred energy state known as the ground state. In this tranquil realm, electrons settle harmoniously into their lowest possible energy levels. For helium, the ground state is a haven where two electrons gracefully twirl within their designated orbitals.
Pauli’s Exclusion Principle: The Electron Matchmaker
The Pauli Exclusion Principle, like a mischievous matchmaker, dictates that no two electrons within an atom can share the same set of four quantum numbers. This means that each electron occupies its own unique energy state, preventing a crowded atomic dance floor.
In helium, the two electrons reside in the lowest energy orbital, the 1s orbital. Each electron spins in opposite directions, satisfying Pauli’s matchmaking rules. This delicate balance ensures that helium’s ground state remains a stable and harmonious haven for its electrons.
Unveiling Helium’s Electron Count
The combination of the ground state concept and Pauli’s Exclusion Principle reveals the number of electrons in helium’s ground state: two. These two electrons, bound together by the attractive force of the nucleus, create a neutral helium atom that floats effortlessly through the cosmic ballet.
Understanding Helium’s Electron Configuration: A Journey into the Realm of Quantum Mechanics
Helium, the second element in the periodic table, holds a unique place in the world of science. Its exceptional properties and electron configuration have captivated scientists for centuries, unlocking insights into the fundamental nature of matter. In this article, we will embark on a journey to unravel the secrets of helium’s electron configuration, exploring its implications on the number of electrons it possesses.
Meet Helium: The Element of Lightness and Inertness
Helium is the lightest and second-most abundant element in the universe. Its unique properties, such as its exceptional lightness and low reactivity, have made it indispensable in various applications, from balloons to MRI scanners.
Atomic Number and Helium’s Identity
Each element is uniquely identified by its atomic number, which represents the number of protons in its nucleus. Helium’s atomic number is 2, indicating the presence of two protons in its atomic nucleus. This information serves as the foundation for understanding helium’s electron configuration.
Electron Configuration: A Blueprint of Electron Arrangement
Electron configuration refers to the distribution of electrons within an atom’s orbitals. It provides a detailed description of the energy levels and the number of electrons occupying each level. Understanding electron configuration is crucial for comprehending an element’s chemical properties and behavior.
Helium’s Electron Configuration: A Simple Yet Profound Arrangement
Helium’s electron configuration is represented as 1s². This notation indicates that helium has two electrons residing in the lowest energy level, denoted as the 1s orbital. The 1s orbital can accommodate a maximum of two electrons, a fundamental principle known as the Pauli Exclusion Principle.
The Pauli Exclusion Principle: A Guiding Force in Quantum Mechanics
The Pauli Exclusion Principle is a cornerstone of quantum mechanics. It postulates that no two electrons within an atom can occupy the same quantum state, defined by a unique combination of energy level, orbital shape, and electron spin. This principle has profound implications for understanding the electron configurations of all elements.
Fermions and Bosons: A Tale of Two Particles
Particles in physics are classified into two categories: fermions and bosons. Fermions, such as electrons, obey the Pauli Exclusion Principle, prohibiting them from sharing the same quantum state. In contrast, bosons, such as photons, are not bound by the Pauli Exclusion Principle and can coexist in the same quantum state.
Helium’s electron configuration, 1s², is a direct consequence of the Pauli Exclusion Principle. This principle dictates that no two electrons within helium can occupy the same quantum state, resulting in two electrons residing in the 1s orbital. Understanding electron configuration provides a deeper appreciation of the unique properties of elements and their behavior in the realm of chemistry.