Diffusion And Effusion: Essential Molecular Movement Processes For Science And Engineering
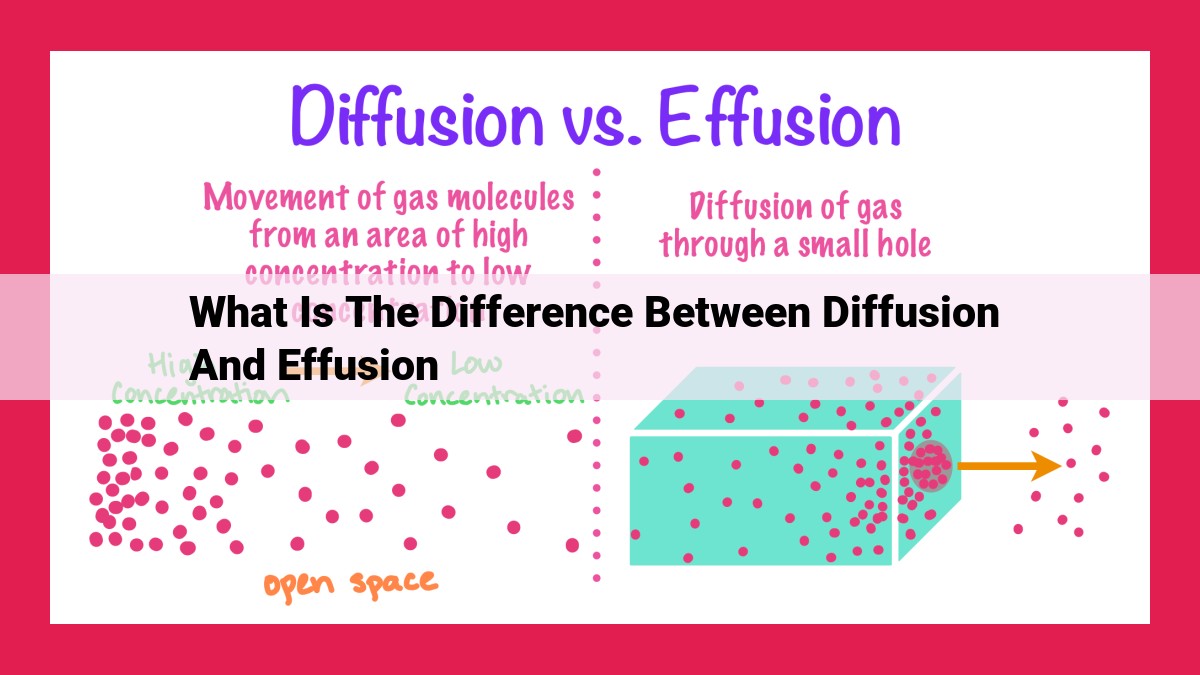
Diffusion and effusion are fundamental processes involving the movement of molecules. Diffusion occurs due to concentration gradients, with molecules moving from high to low concentration areas. It can occur in liquids or gases and is influenced by membrane properties. In contrast, effusion is driven by pressure gradients and is specific to gases. It is insensitive to the medium and follows Graham’s Law of Effusion, which relates effusion rate to molecular mass. Both processes have applications in respiration, drug delivery, gas separation, and leak detection. Understanding these differences is crucial in scientific and practical fields.
Diffusion and Effusion: The Dance of Molecules
In the vast world of science, the movement of molecules plays a crucial role in countless processes. Two fundamental phenomena that govern this movement are diffusion and effusion – processes that are often intertwined but distinctly different in their nature.
Diffusion: A Concentration-Driven Dance
Imagine a room filled with perfume. The molecules of the perfume spread throughout the room, gradually filling every nook and cranny. This is the essence of diffusion, the spontaneous movement of molecules from areas of higher concentration to areas of lower concentration. Driven by the disparity in concentration, molecules embark on a journey, guided by the invisible gradient that leads them towards equilibrium.
Diffusion is not confined to gases alone; it occurs in liquids and even solids. In biological systems, it plays a pivotal role in gas exchange, the transport of nutrients, and the removal of waste products. Cells rely on diffusion to exchange vital substances with their surroundings.
Effusion: A Pressure-Driven Symphony
While diffusion is driven by concentration gradients, effusion is a process that occurs when gases escape through tiny openings. Consider a balloon filled with helium. As the balloon escapes through the opening, the helium molecules stream out, creating a flow. This flow is known as effusion, a process governed by the pressure gradient between the inside and outside of the opening.
Unlike diffusion, effusion is solely a gas-related phenomenon. It finds applications in gas separation, leak detection, and even in space exploration, where it helps scientists study the composition of the atmospheres of distant planets.
A Tale of Two Processes: Diffusion vs Effusion
While both processes involve the movement of molecules, diffusion and effusion are distinctly different.
- Driving Force: Diffusion is driven by concentration gradients, while effusion is driven by pressure gradients.
- Medium: Diffusion can occur in liquids or gases, while effusion is limited to gases.
- Resistance: Diffusion is influenced by membrane properties, while effusion is largely insensitive to the medium.
Harnessing the Power of Molecules
Diffusion and effusion are fundamental processes with a myriad of applications in science and technology. From gas exchange to drug delivery, diffusion plays a crucial role in biological systems. Effusion, on the other hand, finds use in gas separation, leak detection, and even in space exploration. By understanding the intricacies of these molecular movements, scientists and engineers can harness their power to solve problems and advance our knowledge of the world.
Diffusion: The Secret Movement of Molecules
Imagine a swarm of tiny particles drifting through a space like a gentle breeze. This is the essence of diffusion, a fundamental process that drives the movement of molecules in our world. When a substance is present at a higher concentration in one area than another, its molecules naturally diffuse towards the region of lower concentration.
This unstoppable flow is a constant force in our bodies, enabling essential functions like gas exchange in our lungs and the distribution of nutrients in our cells. But what exactly is diffusion?
Defining the Diffusion Dance
Diffusion is the passive movement of molecules down their concentration gradient. This means that molecules drift from a region where they are more concentrated to an area where they are less concentrated. This spontaneous movement occurs without the need for any external energy input.
Related Concepts: Diffusion’s Cousins
Diffusion is closely linked to several other processes:
- Osmosis: The selective diffusion of water across a semipermeable membrane. This allows water to move into or out of cells to maintain proper hydration levels.
- Facilitated diffusion: Diffusion assisted by membrane proteins. These proteins transport specific substances across membranes that are otherwise impermeable.
- Active transport: The movement of molecules against their concentration gradient, requiring energy input. This process is vital for transporting substances into or out of cells against their concentration gradients.
Effusion: Movement of Gases Under Pressure Gradients
In the realm of gases, effusion stands out as a fascinating phenomenon. It’s a process where gases flow from an area of high pressure to low pressure. Unlike diffusion, which involves the movement of molecules in response to concentration gradients, effusion is driven by _pressure gradients.
Definition and Explanation of Effusion
Imagine a container filled with a mixture of gases. When a tiny opening is created in the container, the gas molecules near the opening escape into the surroundings. This escape is known as effusion. The rate of effusion depends on several factors, including the pressure of the gas, the temperature, and the mass of the gas molecules.
Related Concepts
Graham’s Law of Effusion: This law states that the rate of effusion of a gas is inversely proportional to the square root of its molecular mass. In simpler terms, lighter gases effuse faster than heavier gases at the same temperature and pressure.
Knudsen Effusion: This phenomenon occurs when the opening through which the gas effuses is very small. In this case, the gas molecules collide with the opening more often than they collide with each other. As a result, the rate of effusion becomes independent of the molecular mass.
Molecular Flow: At extremely low pressures, the mean free path of gas molecules becomes much larger than the size of the opening. Under these conditions, the gas molecules travel in straight lines and collide with the opening only once. This is known as molecular flow, and the rate of effusion again becomes independent of the molecular mass.
Comparing Diffusion and Effusion: Key Differences
In the realm of science, two fundamental processes govern the movement of molecules: diffusion and effusion. While both involve the motion of molecules, they exhibit distinct characteristics that set them apart.
At their core, diffusion and effusion share a common characteristic: the movement of molecules. However, the driving force behind this movement is where they differ. Diffusion is driven by a concentration gradient, the difference in the concentration of molecules between two regions. Molecules naturally move from areas of high concentration to areas of low concentration, seeking to achieve equilibrium. In contrast, effusion is driven by a pressure gradient, the difference in pressure between two regions. Molecules flow from areas of high pressure to areas of low pressure, propelled by the force of their pressure difference.
Another key difference lies in the medium in which these processes occur. Diffusion can occur in both liquids and gases, whereas effusion is confined to gases alone. In diffusion, molecules encounter resistance as they navigate through the molecular structure of the medium. This resistance influences the rate of diffusion. Conversely, effusion is insensitive to the medium, as molecules flow freely through empty space.
Finally, diffusion and effusion differ in their response to physical barriers. Diffusion is hindered by the presence of membranes, selectively permeable barriers that restrict the passage of certain molecules. The properties of these membranes can influence the rate of diffusion, depending on the type of molecule and the nature of the membrane. In contrast, effusion is unaffected by the presence of membranes, as molecules simply flow through any available space.
Diffusion and Effusion: Unraveling the Movement of Molecules
In the world of science, diffusion and effusion are fundamental processes that govern the movement of molecules, shaping everything from biological functions to industrial applications.
Diffusion: The Dance of Molecules
Imagine a crowded room where molecules are bustling about like tiny dancers. Diffusion is the relentless movement of these molecules from areas of high concentration to areas of low concentration. It’s like a cosmic ballet, where molecules glide effortlessly down their own concentration gradients.
Diffusion has a myriad of biological implications. It facilitates gas exchange in respiration, allowing oxygen to enter our lungs and carbon dioxide to escape. It also plays a crucial role in drug delivery, ensuring that medications can reach their intended targets within our bodies.
Effusion: A Gaseous Escape
Now, picture a gas trapped within a container. Effusion is the escape of these gas molecules through tiny openings, driven by pressure gradients. Unlike diffusion, effusion is only possible in gases and is insensitive to the medium through which the gas flows.
Effusion has found practical applications in gas separation and leak detection. By controlling the size of openings, we can selectively filter gases based on their molecular masses. Moreover, effusion can be used to detect leaks in pipes and containers by monitoring the escape rate of gases.
The Distinctive Differences: Diffusion vs. Effusion
While both diffusion and effusion involve molecular movement, they differ in several key aspects:
- Driving force: Diffusion is driven by concentration gradients, while effusion is driven by pressure gradients.
- Medium: Diffusion can occur in liquids or gases, while effusion is limited to gases.
- Resistance: Diffusion is influenced by membrane properties, while effusion is largely insensitive to the medium.
Understanding these differences is crucial for comprehending the diverse scientific and practical applications of diffusion and effusion. From gas exchange in our bodies to leak detection in industries, these fundamental processes shape our world in countless ways.