Determining The Oxidation Number Of Manganese In Potassium Permanganate (Kmno4): A Comprehensive Guide
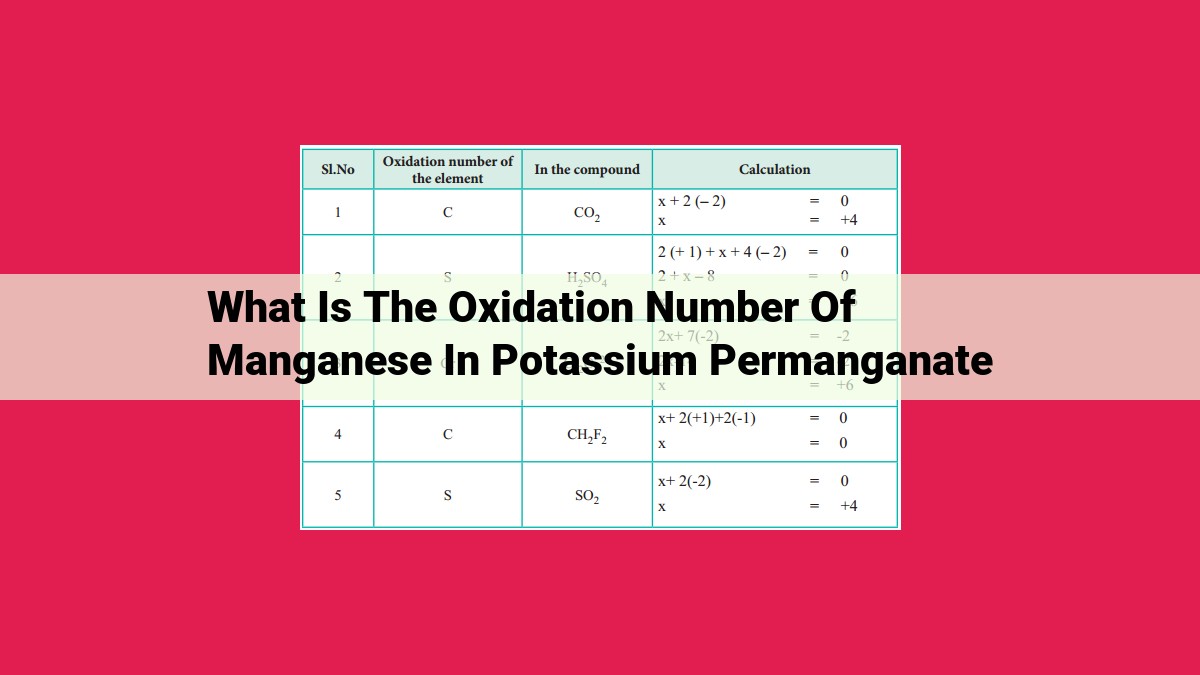
The oxidation number of manganese in potassium permanganate (KMnO4) can be calculated using the oxidation numbers of potassium (+1), oxygen (-2), and the overall charge of the compound (-1). By assigning an oxidation number of x to manganese, we can solve for x using the equation: (+1) + 4(-2) + x = -1. This gives us an oxidation number of +7 for manganese in potassium permanganate.
What is Oxidation Number?
- Explain the concept of oxidation number as the hypothetical charge of an atom in a compound.
- Discuss how oxidation number is calculated based on valence electrons and electronegativity.
What is Oxidation Number?
Let’s embark on an intriguing adventure to decipher the enigma of oxidation numbers. Picture an atom residing within a molecule, its electrons fervently dancing around its nucleus. The oxidation number is a brilliant concept that assigns a hypothetical charge to this atom, reflecting its perceived charge if all bonds were ionic rather than covalent.
This ingenious scheme is meticulously calculated using valence electrons and electronegativity. Valence electrons, residing in the outermost shell of an atom, play a pivotal role in chemical bonding. Electronegativity, a measure of an atom’s affinity for electrons, dictates the electron distribution within bonds. By considering these factors, we can unravel the oxidation number of an atom, providing us with a profound understanding of its chemical behavior.
Understanding Redox Reactions
In the realm of chemistry, redox reactions dance elegantly, weaving a tale of electron exchange and chemical transformation. Redox, short for reduction-oxidation, describes reactions where electrons frolic, eagerly jumping from one atom to another, forging new bonds and breaking old ones.
Oxidation is the act of an atom losing electrons, like a shy debutante shedding her inhibitions on the dance floor. Reduction, on the other hand, is the reverse – an atom gaining electrons, mirroring the excitement of an exuberant reveler embracing the party’s energy.
Redox reactions are the lifeblood of chemical processes, the driving force behind countless reactions in nature, industry, and even our very bodies. Understanding them is akin to unriddling the secrets of the universe, unlocking the mysteries that govern the ebb and flow of matter.
Role of Oxidation Numbers:
Oxidation numbers are the gatekeepers of redox reactions, numerical values that describe the hypothetical charge of an atom in a compound. They reveal the electronic intentions of atoms, indicating their willingness to lose or gain electrons.
In the grand dance of redox, oxidation numbers guide the waltz of electrons, dictating which atoms surrender and which embrace. They provide a roadmap for understanding the electron transfer that orchestrates these chemical transformations.
Potassium Permanganate: A Versatile Oxidizing Agent
Imagine a vibrant purple liquid that’s not just eye-catching but also a powerful tool in the world of chemistry. Meet potassium permanganate, a chemical compound with remarkable oxidizing properties.
What is Potassium Permanganate?
Potassium permanganate is a salt composed of potassium, manganese, and oxygen atoms. It’s known for its intense purple color in aqueous solutions, which is caused by the manganese ions’ unique electronic structure.
Oxidation Potential and Redox Reactions
Potassium permanganate’s oxidizing power stems from its ability to readily accept electrons. When it reacts with other substances, it undergoes reduction, meaning it gains electrons. Simultaneously, the other substance undergoes oxidation, transferring electrons to the permanganate ion. This exchange of electrons is what drives redox reactions.
Practical Applications in Oxidation Reactions
Potassium permanganate finds wide application in various industries由于其氧化性能.
-
Water Treatment: It’s used as a disinfectant and deodorizer, removing impurities and unpleasant odors from water.
-
Chemical Analysis: Potassium permanganate serves as a titrant in redox titrations, where it’s used to determine the concentration of reducing agents.
-
Oxidizing Agent in Organic Chemistry: It’s employed to oxidize alkenes, alcohols, and other organic compounds, converting them into more functionalized products.
Role of Oxidation Number
Understanding oxidation numbers is crucial for comprehending redox reactions involving potassium permanganate. Oxidation number represents the hypothetical charge of an atom within a compound. By calculating the oxidation numbers of the reactants and products, we can track the flow of electrons and determine the changes that occur during the reaction.
In conclusion, potassium permanganate is a versatile oxidizing agent with a wide range of applications in various fields. Its characteristic purple color, coupled with its ability to facilitate electron transfer and undergo redox reactions, makes it a valuable tool for both analytical and synthetic chemistry.
Manganese: The Transition Metal with Multifaceted Oxidation States
Meet Manganese, a fascinating transition metal that boasts an extraordinary ability to adopt diverse oxidation states. These states play a pivotal role in defining its chemical demeanor.
Unlike ordinary metals, manganese possesses a unique characteristic: it can readily lose or gain electrons, giving rise to a spectrum of oxidation states. This versatility empowers manganese to participate in a captivating array of chemical reactions, endowing it with remarkable versatility.
Oxidation States are numerical representations of the hypothetical charge an atom would have if all its bonds were purely ionic. Understanding these states is paramount in unraveling the intricate world of manganese’s chemical behavior. For instance, in the compound potassium permanganate (KMnO4), manganese exists in the +7 oxidation state, indicating its high tendency to accept electrons and act as an oxidizing agent.
Conversely, in another compound, manganese(II) chloride (MnCl2), manganese dons the +2 oxidation state, showcasing its willingness to donate electrons and behave as a reducing agent. These contrasting oxidation states highlight manganese’s adaptability and its pivotal role in various chemical processes.
As a transition metal, manganese occupies a special place in the periodic table. Transition metals are renowned for their ability to form stable ions with multiple oxidation states, making them indispensable components in countless industrial and biological processes. Manganese, with its diverse oxidation states, is no exception.
Therefore, understanding the oxidation states of manganese is not merely an academic pursuit but an essential key to unlocking the secrets of its chemical behavior. By unraveling these states, we gain a deeper appreciation for the intricate tapestry of chemical reactions that shape our world.
Calculating the Oxidation Number of Manganese in Potassium Permanganate
Step 1: Understanding Oxidation Numbers
In the world of chemistry, every atom carries a hypothetical charge known as its oxidation number. This number represents the atom’s perceived electron distribution in a compound.
Step 2: The Special Case of Potassium Permanganate
Let’s focus on a particular compound: potassium permanganate (KMnO₄). This compound is a powerful oxidizing agent, meaning it has the ability to transfer electrons to other substances. To fully understand its role in these reactions, we need to determine the oxidation number of manganese (Mn) in KMnO₄.
Step 3: Assembling the Puzzle
The oxidation number of manganese is the key piece in this puzzle. To calculate it, we’ll use a simple formula based on the oxidation numbers of the other atoms in the compound.
Potassium (K): Potassium is a metal with a fixed oxidation number of +1.
Oxygen (O): Oxygen typically has an oxidation number of -2, unless it’s bonded to a more electronegative element like fluorine.
Overall Charge of KMnO₄: The compound as a whole carries a negative charge of -1.
The Magic Formula:
Now we have the ingredients for our calculation:
(1 × +1) + (4 × -2) + Oxidation Number of Manganese = -1
Solving for Manganese:
Solving for the unknown Oxidation Number of Manganese, we get:
(+1) + (-8) + Oxidation Number of Manganese = -1
Oxidation Number of Manganese = +7
What Does It Mean?
The oxidation number of manganese in KMnO₄ is +7, indicating that each manganese atom has effectively lost seven electrons. This high oxidation state makes potassium permanganate a potent oxidizer, ready to donate these electrons to other substances in redox reactions.
Applications of Potassium Permanganate in Oxidation Reactions
Potassium permanganate, a widely used chemical compound, plays a crucial role as an oxidizing agent in various practical applications. Its strong oxidizing properties make it a valuable reagent in many chemical processes.
One notable application of potassium permanganate is in disinfection. Its bactericidal properties allow it to effectively kill bacteria and other microorganisms. It is utilized in water purification systems to ensure safe drinking water. Furthermore, it finds use in sanitizing swimming pools and other water bodies.
Potassium permanganate is also employed in analytical chemistry. In titrations, it serves as an oxidizing agent to determine the concentration of reducing agents. This technique helps quantify the amount of a substance in a solution. Additionally, it is used in qualitative analysis to identify specific ions and compounds.
In organic chemistry, potassium permanganate is a powerful oxidant used in various reactions. It can oxidize alkenes to form glycols, alcohols to aldehydes or ketones, and sulfides to sulfoxides. These reactions are essential in the synthesis of complex organic molecules.
Potassium permanganate’s versatility as an oxidizing agent makes it a valuable tool in numerous applications. Its strong oxidizing properties enable it to disinfect, analyze, and synthesize various chemical compounds. Understanding the oxidation number of potassium permanganate is crucial for predicting its reactivity and understanding the mechanisms behind its oxidizing actions.