How To Determine Initial Concentration: A Comprehensive Guide With Precise Calculations
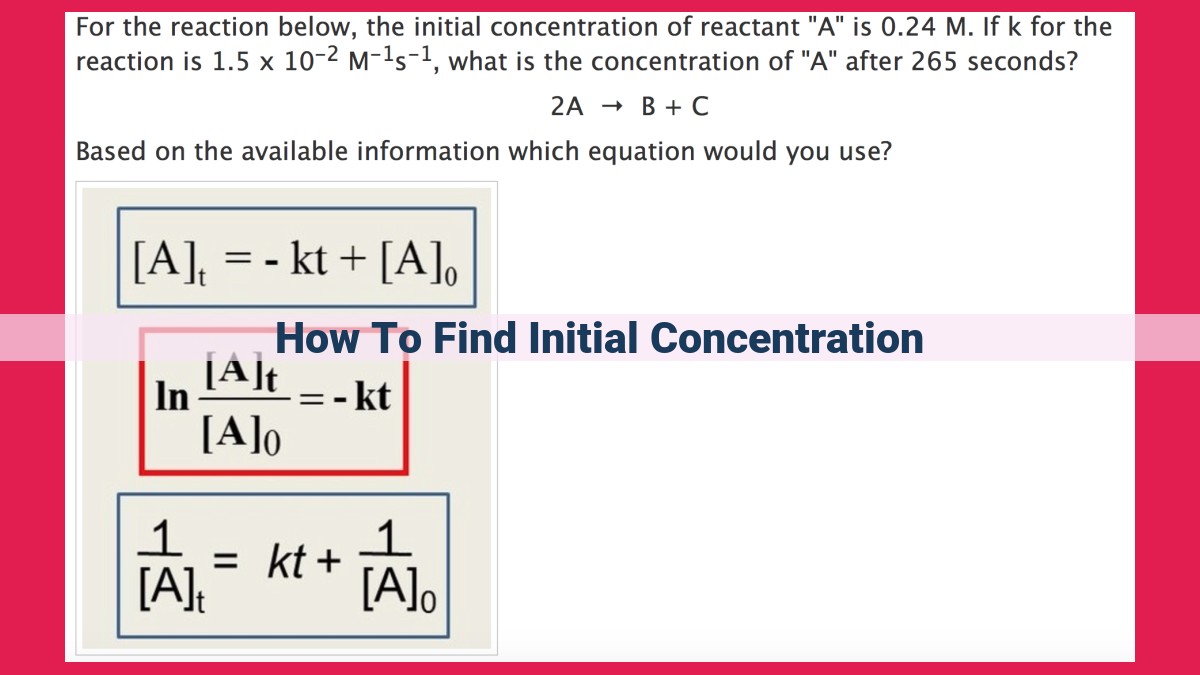
To find initial concentration, use the formula: Initial Concentration = Amount of Substance / Volume of Solution. Determine the amount of substance using mass, molarity, or moles. Calculate the volume of solution using the graduated cylinder or flask measurements. Substitute these values into the formula to find the initial concentration. Ensure accurate measurements for reliable results.
Unveiling the Importance of Initial Concentration: A Comprehensive Guide
In the realm of science and practical applications, understanding initial concentration holds immense significance. It refers to the concentration of a substance at the start of a reaction, process, or experiment. Determining initial concentration accurately is pivotal, as it paves the way for reliable results and informed decision-making in various fields.
Importance of Initial Concentration
Initial concentration plays a crucial role in diverse areas, including:
-
Chemical Reactions: Understanding the initial concentrations of reactants is essential for predicting reaction rates, product yields, and equilibrium positions.
-
Analytical Chemistry: Determining the initial concentration of an analyte in a sample is crucial for quantitative analysis, environmental monitoring, and forensic investigations.
-
Environmental Monitoring: Measuring initial concentrations of pollutants in air, water, and soil is vital for assessing environmental health and implementing remediation strategies.
-
Pharmacology: Understanding the initial concentration of drugs in the body helps optimize dosage regimens, minimize side effects, and maximize therapeutic efficacy.
The Need for Accurate Determination
Accurate determination of initial concentration is imperative for reliable and meaningful results. Errors in initial concentration can lead to:
- Misinterpretation of experimental data
- Incorrect prediction of reaction outcomes
- Inaccurate environmental assessments
- Suboptimal drug treatments
Initial concentration is a fundamental concept with far-reaching implications in science and practical applications. Its accurate determination is crucial for ensuring reliable results and informed decision-making. Understanding the importance of initial concentration and employing appropriate methods for its determination are essential skills for scientists, researchers, and anyone seeking to delve into the intricacies of various fields.
Formula and Calculations for Initial Concentration
In the realm of chemistry and science, understanding the concept of initial concentration is paramount. It represents the concentration of a substance at the onset of a reaction or experiment. Accurately determining initial concentration is vital for a plethora of applications, including chemical reactions, analytical chemistry, and environmental monitoring.
The formula for initial concentration is expressed as:
**Initial Concentration (C<sub>i</sub>) = Amount of Substance (n) / Volume of Solution (V)**
where:
- Ci is the initial concentration in moles per liter (M)
- n is the amount of substance in moles (mol)
- V is the volume of the solution in liters (L)
This formula is derived from the definition of concentration, which is the amount of substance per unit volume. By dividing the amount of substance by the volume of the solution, we obtain the concentration of the substance in that solution.
Units of measurement for concentration are typically expressed in moles per liter (M), millimoles per liter (mM), or micromoles per liter (µM). The amount of substance refers to the number of moles of the substance present in the solution, while the volume of solution indicates the total volume of the solution in liters.
Understanding the relationship between concentration and the amount of substance is crucial. The concentration of a solution is directly proportional to the amount of substance dissolved in the solution. This means that as the amount of substance increases, the concentration of the solution also increases. Conversely, as the amount of substance decreases, the concentration of the solution decreases.
By employing the formula and understanding the concepts behind initial concentration, scientists and researchers can accurately determine the initial concentration of a substance in a solution. This information serves as a foundation for various scientific endeavors, enabling us to unravel the mysteries of the chemical world and make informed decisions in practical applications.
Steps to Find Initial Concentration: A Comprehensive Guide
In the realm of scientific exploration and analytical endeavors, understanding the initial concentration of a solution is paramount. It unveils the path to unraveling mysteries, unlocking insights, and making informed decisions. The following comprehensive guide will illuminate the step-by-step procedure to find initial concentration, empowering you to embark on your scientific quests with precision and confidence.
Firstly, the formula for initial concentration reigns supreme:
Initial Concentration (Ci) = Amount of Substance (n) / Volume of Solution (V)
This straightforward formula holds the key to determining the initial concentration of a solution.
-
Measure the Amount of Substance (n):
The amount of substance represents the quantity of a chemical species present in the solution. Units like moles (mol) are commonly employed to express this. Precisely measure the mass or volume of the substance using calibrated equipment, such as a weighing balance or graduated cylinder. Convert these values to moles using the substance’s molar mass or density.
-
Determine the Volume of Solution (V):
Accurately measure the volume of the solution using appropriate glassware like a volumetric flask or burette. The volume should be expressed in units of liters (L).
-
Substitute Values and Calculate Ci:
Plug the measured amount of substance and volume of solution into the formula: Ci = n/V. Perform the calculation to obtain the initial concentration of the solution.
-
Consider Specific Methods for Different Solutions:
Tailor your approach based on the type of solution you’re dealing with:
-
Solid Solutions: Dissolve the solid in a solvent to create a homogeneous mixture.
-
Liquid Solutions: Directly measure the volume of the liquid and proceed with calculations.
-
Gaseous Solutions: Determine the mass and volume of the gas to calculate its initial concentration.
Remember, meticulous adherence to these steps and employing reliable equipment is crucial for accurate initial concentration determination. The results of your scientific endeavors depend on the precision of your measurements.
Essential Role of Initial Concentration in Diverse Applications
Understanding the Significance of Initial Concentration
Initial concentration, a crucial parameter, plays an indispensable role in countless fields, including chemistry, biochemistry, environmental science, and medicine. It represents the starting concentration of a substance in a solution or mixture. Accurately determining initial concentration is paramount for various applications, as it serves as a critical input for calculations, modeling, and decision-making.
Chemical Reactions: Predicting Reactivity
In chemical reactions, initial concentration significantly influences the reaction rate and extent. By knowing the initial concentrations of reactants, scientists can predict the rate at which the reaction proceeds and calculate the amount of products formed. This knowledge is crucial for designing efficient chemical processes, optimizing reaction conditions, and understanding reaction mechanisms.
Analytical Chemistry: Quantifying Unknown Solutions
Initial concentration is a fundamental parameter in analytical chemistry, where the concentration of an unknown sample needs to be determined. Analytical techniques, such as titrations and spectrophotometry, rely on precise initial concentration measurements to accurately quantify the unknown analyte.
Environmental Monitoring: Assessing Pollution Levels
In environmental monitoring, initial concentration is crucial for assessing pollution levels in various environmental compartments, such as air, water, and soil. By measuring the initial concentration of pollutants, scientists can track their sources, monitor their dispersion, and evaluate their potential impacts on human health and ecosystems.
Initial concentration is a cornerstone parameter in a wide range of scientific fields, including chemistry, analytical chemistry, and environmental monitoring. Accurately determining initial concentration is essential for understanding reaction rates, quantifying unknown solutions, and assessing pollution levels. Its applications are far-reaching, impacting various aspects of our lives and the environment.
Advantages and Disadvantages of the Initial Concentration Formula
In the realm of chemistry and beyond, understanding initial concentration is crucial. The formula for calculating initial concentration offers a valuable tool for determining the starting point of reactions, analyzing solutions, and monitoring environmental parameters. However, like any scientific concept, this formula has its own strengths and limitations.
Advantages:
- Accuracy: When applied correctly, the initial concentration formula provides a precise estimation of the initial concentration of a solution.
- Simplicity: The formula is straightforward and easy to understand, making it accessible to students and researchers alike.
- Wide Applicability: The formula has universal application across various fields, including chemistry, biology, and environmental science.
Disadvantages:
- Errors: As with any formula, the accuracy of the initial concentration calculation depends on the precision of the measurements used. Errors in measuring volume or mass can lead to incorrect results.
- Assumptions: The formula assumes that the solution is homogeneous and that the reaction or process under consideration follows linear kinetics. Deviations from these assumptions can introduce errors.
- Limitations in Complex Mixtures: In complex mixtures where multiple substances are present, determining the initial concentration of a specific component can be challenging using this formula alone.
Despite these limitations, the formula for initial concentration remains an essential tool for scientists and researchers. By understanding its advantages and disadvantages, users can apply it effectively and minimize the potential for errors.
Ensure Accurate Initial Concentration Determination
Determining the initial concentration is crucial for countless scientific and practical applications. To guarantee precise results, implement these invaluable tips:
-
Utilize **Precise Measurement Techniques: Employ calibrated equipment and meticulously measure volumes and masses to avoid errors.
-
Verify Data Diligently: Conduct multiple measurements and calculate the average to minimize uncertainties and enhance reliability.
-
Minimize Systematic Errors: Strictly adhere to established protocols and calibrate equipment regularly to eliminate systematic errors.
-
Check for Contamination: Ensure glassware and reagents are free from impurities that could alter initial concentration.
-
Use Reliable Equipment: Invest in high-quality equipment designed for precise measurements. Malfunctioning or outdated devices can lead to inaccurate results.
Remember, accurate initial concentration determination empowers you with reliable data for informed decisions and successful outcomes.
Example Calculations and Applications
- Present worked-out examples to demonstrate the application of the formula in different scenarios.
- Show how initial concentration is used in practical situations, such as preparing solutions for experiments or analyzing samples.
Example Calculations and Applications: Demystifying Initial Concentration
Initial concentration plays a crucial role in scientific and practical endeavors. Let’s delve into some real-world examples to illustrate its significance.
In a chemistry lab, you need to prepare a solution of sodium chloride for an experiment. The recipe calls for a 0.1 molar (M) solution, which means there should be 0.1 moles of sodium chloride dissolved in every liter of solution. Using the initial concentration formula, you can determine how much salt to dissolve:
Initial Concentration (M) = Moles of Solute / Volume of Solution (L)
Suppose you have a 500 ml flask. Rearranging the formula, we get:
Moles of Solute = Initial Concentration (M) * Volume of Solution (L)
Plugging in the values:
Moles of Solute = 0.1 M * 0.5 L = 0.05 moles
Now you know that you need to dissolve 0.05 moles of sodium chloride in 500 ml of water to create a 0.1 molar solution.
In environmental monitoring, initial concentration is critical for assessing pollutant levels. Scientists measure the initial concentration of pollutants, such as heavy metals or organic compounds, in environmental samples like water or soil. Comparing these concentrations to established standards helps determine the extent of pollution and the potential risks it poses.
For instance, let’s say the acceptable limit for lead in drinking water is 0.015 mg/L. If a water sample shows an initial concentration of 0.02 mg/L, it exceeds the safe limit and requires further investigation and remediation.
These examples highlight the diverse and crucial applications of initial concentration. Whether it’s preparing solutions for experiments, analyzing samples, or assessing environmental health, understanding and accurately determining initial concentration is essential for scientific accuracy and practical decision-making.
Special Considerations for Complex Mixtures
Determining initial concentration in solutions with multiple known components is straightforward. However, when dealing with complex mixtures of unknown compositions, the task becomes more challenging.
In these scenarios, alternative approaches are necessary to resolve and analyze the mixture’s constituents. Chromatography and spectroscopy are two powerful techniques commonly employed for this purpose.
Chromatography involves separating the mixture’s components based on their different physical or chemical properties. By passing the mixture through a stationary phase, the components migrate at varying rates, allowing their separation and identification.
Spectroscopy, on the other hand, utilizes the interaction of electromagnetic radiation with the mixture’s components to obtain information about their molecular structure and concentration. Different spectroscopic techniques, such as UV-Vis spectroscopy and mass spectrometry, provide valuable insights into the mixture’s composition.
By combining these techniques, scientists can resolve complex mixtures and determine the initial concentration of individual components. This information is essential in various fields, including environmental monitoring, drug discovery, and forensic science.