Understanding Deposition: Direct Gas-To-Solid Transformation And Its Significance
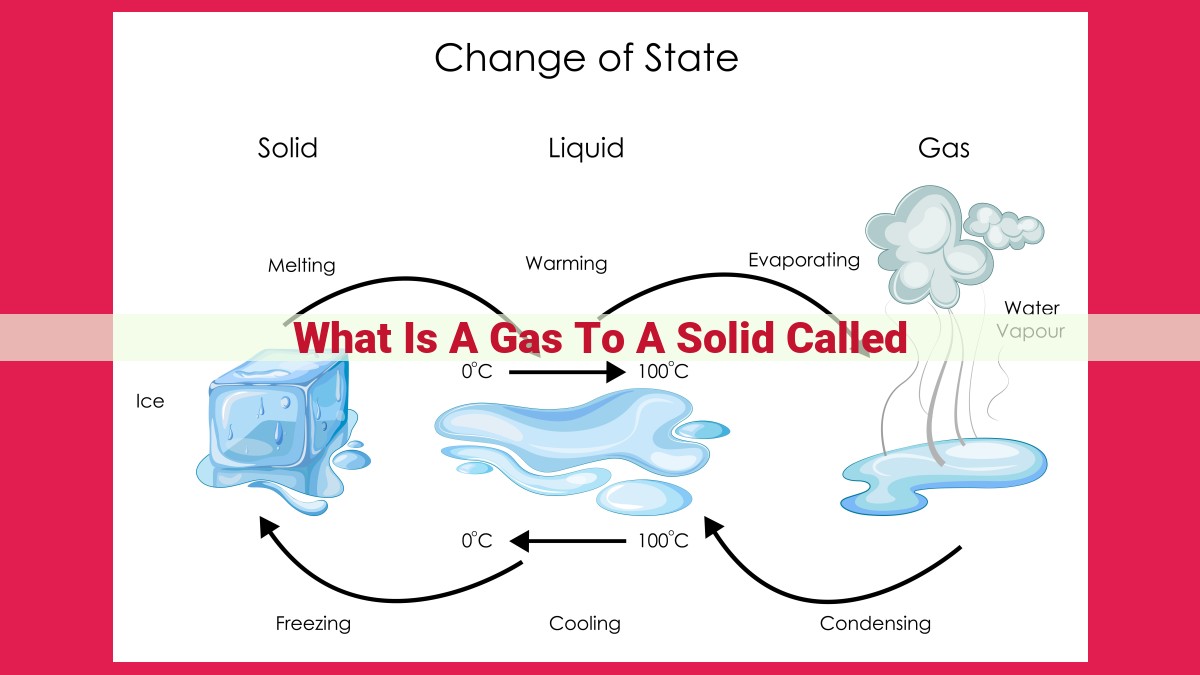
Deposition is the conversion of a gas directly into a solid, bypassing the liquid phase. It occurs when gas particles lose energy and slow down, allowing them to form solid crystals on surfaces. Unlike condensation, which involves gas transforming into liquid, deposition does not require a liquid intermediate. Related concepts include sublimation (direct transition from solid to gas), condensation (gas to liquid), liquefaction (gas to liquid), and various phase change processes like evaporation, boiling, and solidification. Understanding these concepts provides insight into the behavior of matter and the changes it undergoes in different conditions.
Deposition: The Transformation of Gas into Solid
In the realm of matter, we witness a fascinating dance of transformations, where substances seamlessly shift between states of existence. One such metamorphosis is deposition, the enigmatic conversion of gas to solid.
Imagine a pristine winter morning, where the air hangs heavy with frigid vapors. As these vapors brush against the icy surfaces of trees or buildings, they undergo a remarkable alchemy. They condense, transforming into tiny ice crystals that adorn the landscape with a shimmering, ethereal beauty. This phenomenon is deposition.
Understanding Deposition
Deposition is the direct transition from gas to solid, bypassing the intermediate liquid state. It occurs when a gas rapidly cools to a temperature below its condensation point, the point at which it would normally condense into a liquid. This rapid cooling process prohibits the formation of liquid droplets, resulting in the direct formation of solids.
Interestingly, deposition is exothermic, meaning it releases heat into the surroundings. This heat release can create a localized increase in temperature, further facilitating the deposition process.
Delving into the Intricate World of Phase Transitions: Related Concepts to Deposition
When we think about phase transitions, we often picture the familiar sight of water turning into ice or steam. But these dramatic transformations are just a glimpse into the fascinating realm of these physical processes. Deposition, the conversion of a gas directly into a solid, plays a crucial role in shaping our world. To fully grasp deposition, it’s essential to explore its closely related concepts.
Condensation: The Liquid Intermediary
Condensation, the transition from a gas to a liquid, is an intimate dance between molecules. As a gas cools, its molecules lose kinetic energy, slowing down and colliding more frequently. When these collisions become frequent enough, the molecules begin to cling together, forming tiny droplets of liquid.
This process is ubiquitous in our daily lives. Our breath condenses on cold windows, turning into tiny droplets of moisture. Rain, snow, and fog are all manifestations of condensation, evidence of the water vapor in the air transforming into visible liquid.
Liquefaction: The Melting Moment
Liquefaction, the process by which a gas transforms into a liquid, is a bit more forceful than condensation. Here, the gas is subjected to high pressure, compressing its molecules into a liquid state. This process is used industrially to produce liquefied petroleum gas (LPG), which is a convenient fuel for transportation and cooking.
Sublimation: A Direct Leap
Sublimation, the direct transformation of a substance from a gas to a solid, bypasses the liquid phase entirely. This phenomenon is often observed in frozen environments, where water vapor in the air crystallizes directly into snowflakes or frost.
The process of sublimation can also be harnessed for industrial applications. Dry ice, for instance, is produced by sublimating carbon dioxide gas into solid form. This remarkable substance has numerous applications, from food preservation to medical procedures.
Understanding the Dance of Phase Transitions
These related concepts are intricately connected, forming a web of phase transitions that shape the world around us. By understanding these processes, we gain a deeper appreciation for the dynamic nature of matter and the forces that govern its behavior.
Condensation: The Liquid Transformation of Gases
As we gaze up at the sky on a cloudy day, we witness the marvelous spectacle of condensation – the magical conversion of water vapor into liquid droplets. This everyday phenomenon is a fascinating example of how matter changes phases in our world.
Condensation occurs when gases cool below their dew point, the temperature at which they become saturated and cannot hold any more water vapor. As the gas cools, its particles lose energy and slow down, causing them to condense into tiny liquid droplets. These droplets then coalesce to form clouds, fog, or dew.
This process of ****condensation is not limited to water vapor. Many other gases, such as carbon dioxide and ammonia, can also condense into liquids under the right conditions. In fact, condensation is an integral part of many industrial processes, such as the refining of petroleum and the production of chemicals.
In addition to condensation, there are several other key phase changes that involve gases. Evaporation is the opposite of condensation, where a liquid transforms into a gas. Boiling occurs when a liquid reaches its boiling point and rapidly vaporizes. ****Precipitation** is the process by which water vapor in the atmosphere condenses into clouds and falls to the ground as rain, snow, or hail.
Understanding these interrelated concepts is crucial for comprehending the dynamic nature of our planet and the myriad ways in which matter transforms around us. By studying condensation and its related phase changes, we gain a deeper appreciation for the intricate tapestry of our physical world.
Liquefaction: The Enchanting Transformation of Gas to Liquid
In the kaleidoscope of phase changes, the metamorphosis of gas into liquid holds a special allure known as liquefaction. It’s a captivating process where the intangible becomes tangible, a misty essence coalescing into a shimmering fluid.
Melting and Fusion: The Symphony of Solids and Liquids
Liquefaction’s celestial dance begins with melting, the graceful transition of a solid into a liquid. Imagine an ice cube basking in the warmth of the sun, its crystalline structure surrendering to the gentle allure of heat. As it melts, the bonds holding its molecules in place loosen, allowing them to flow freely like a gentle stream.
Solidification: The Liquid’s Journey Back to Solidity
The saga continues with solidification, the reciprocal of melting, where liquid transforms back into solid. As temperatures drop, the liquid’s molecules slow their frenetic movement and begin to cling to each other, forming an intricate crystalline lattice. This process, a testament to nature’s symmetry, brings the liquid’s fluidity to a standstill.
Liquefaction: Uniting the Elements of Air and Water
Liquefaction itself, the enchanting union of gas and liquid, often occurs under extreme conditions. By subjecting gases to high pressures and low temperatures, we can witness the remarkable transformation of invisible air into tangible liquid. This process finds its practical application in industries where gases such as oxygen, nitrogen, and hydrogen are liquefied for storage and transportation.
With its captivating allure and practical applications, liquefaction stands as a testament to the dynamic nature of matter. It’s a symphony of changes, where the ethereal and the tangible intertwine, reminding us of the boundless possibilities that lie within the world of science.
Sublimation: The Enigmatic Phase Transition
In the realm of matter, where substances dance and transform, there exists a captivating process called sublimation. Unlike its more well-known counterparts, condensation and liquefaction, sublimation defies convention by allowing a substance to skip the liquid phase altogether, transitioning directly from gas to solid.
Imagine a pristine winter’s day, where the air is crisp and the ground is cloaked in a blanket of snow. As the sun’s rays peek through the clouds, the snow begins to sparkle and vanish, seemingly evaporating into thin air. This enchanting spectacle is a testament to the power of sublimation.
The Dance of Molecules
Sublimation occurs when the molecules of a substance gain enough energy to overcome the bonds that hold them together in the solid state. As these molecules break free, they escape into the surrounding space, where they float as individual gas particles.
A Two-Way Street
Just as a solid can sublime into a gas, a gas can also undergo the reverse process, known as deposition. This occurs when gas molecules collide with a cold surface and lose energy, causing them to condense directly into a solid.
Notable Examples
Sublimation is a common phenomenon that has countless applications in everyday life. For instance:
- Dry ice sublimes directly from a solid to a gas, making it ideal for creating fog effects and keeping perishable goods cold.
- Perfumes contain volatile substances that sublime at body temperature, releasing their alluring scents.
- Snow sublimes from the ground on cold, dry days, contributing to the formation of beautiful snow crystals.
A Key Concept in Phase Changes
Understanding sublimation is crucial for comprehending the diverse ways in which substances can change their physical states. By exploring the principles of sublimation, we gain a deeper appreciation for the intricate dynamics of matter and the ever-changing symphony of nature.