Unlocking The Secrets Of Decay Constants: Applications And Calculation Methods
To find the decay constant, one can utilize different methods. Using half-life (t½), the formula λ = ln(2) / t½ is employed, where λ is the decay constant. Alternatively, with activity (A) and using the equation A = -λN, where N is the number of decaying atoms, λ can be calculated. Furthermore, the decay rate (R) can be used via the formula R = -λN, where R represents the fractional decay over time. Understanding exponential decay, expressed as N(t) = N0e^(-λt), is crucial, where N0 is the initial atom count and t is the elapsed time. Decay constants find applications in various fields, including carbon dating, medical imaging, and nuclear engineering.
Understanding Radioactive Decay: Unveiling the Secrets of Atomic Transformations
In the realm of science, unraveling the mysteries of the atomic world has led to groundbreaking discoveries. One such phenomenon is radioactive decay, a process that has captivated the imagination of scientists and shaped our understanding of the universe.
Radioactive decay, simply put, is the disintegration of unstable atomic nuclei, releasing energy and transforming into more stable isotopes. This remarkable process holds immense significance, as it serves as a natural clock in numerous scientific fields, from unraveling the secrets of ancient artifacts to advancing medical diagnostics and safeguarding nuclear facilities.
To comprehend radioactive decay, we must first grasp some fundamental concepts:
- Half-life (t½): The period required for half of the unstable atoms in a sample to decay. This numeric value provides a measure of how quickly a radioactive substance loses its radioactivity.
- Activity (A): The rate at which radioactive decay occurs, measured in decays per second. It indicates the number of atoms in the sample that are disintegrating at any given moment.
- Decay rate (R): The fraction of atoms decaying per unit time, expressed as a percentage. This value quantifies the pace at which radioactivity decreases.
- Exponential decay: The pattern of radioactive decay, in which the number of radioactive atoms remaining decreases exponentially over time. This behavior is captured by the equation: N(t) = N0e^(-λt), where N(t) represents the number of atoms at time t, N0 is the initial number of atoms, λ is the decay constant, and t is the elapsed time.
Finding the Decay Constant Using Half-life
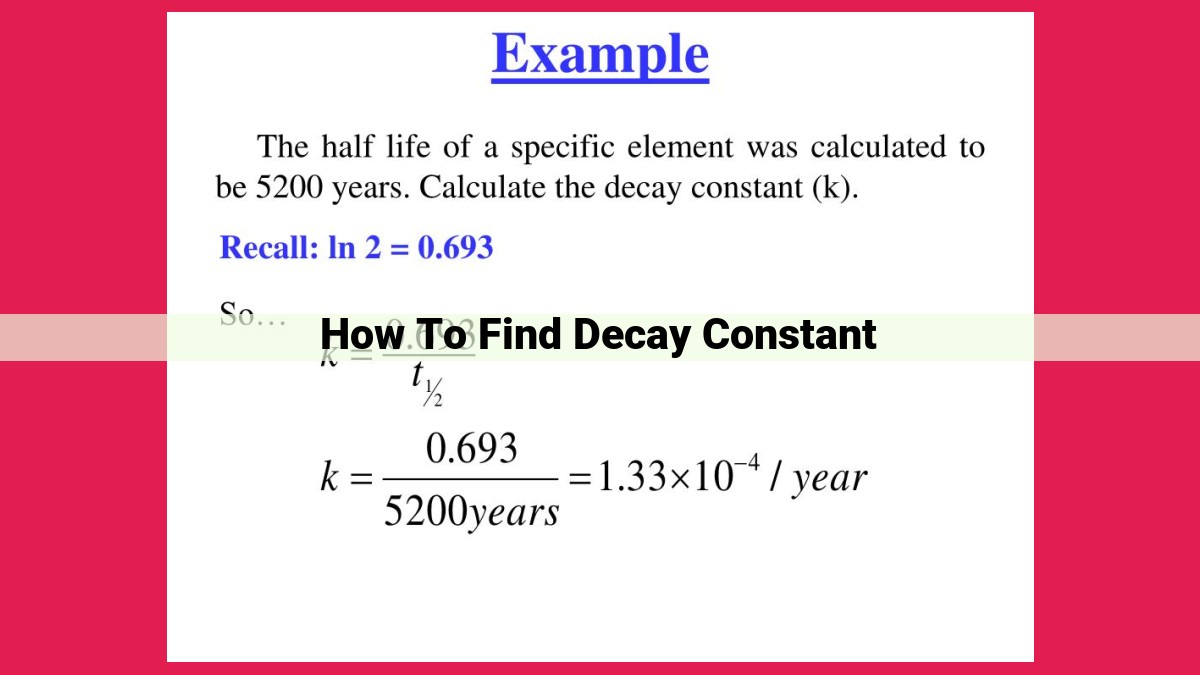
In the realm of radioactive decay, understanding the decay constant, denoted by the Greek letter lambda (λ), is crucial. This constant governs the rate at which radioactive atoms disintegrate over time. One key aspect of decay constants is their inverse relationship with half-life, a fundamental concept in radioactivity.
Half-life and Its Significance
Half-life, represented by the symbol t½, is the time it takes for half of a radioactive sample to decay. This means that after one half-life, only half of the original atoms remain. The inverse relationship between half-life and decay constant implies that a shorter half-life corresponds to a higher decay constant and vice versa.
Formula: λ = ln(2) / t½
The formula that quantifies this inverse relationship is:
λ = ln(2) / t½
where:
- λ is the decay constant
- t½ is the half-life
This formula can be derived from the exponential decay law, which describes the decrease in the number of radioactive atoms over time. By taking the natural logarithm of both sides of this law and setting the time equal to the half-life, we arrive at the formula above.
Practical Applications
The relationship between decay constant and half-life is not just a theoretical concept; it has practical applications in various fields, including:
- Radioactive dating: By measuring the ratio of radioactive isotopes to their stable counterparts in geological samples, scientists can determine the age of ancient artifacts or fossils.
- Medical imaging: Radioactive isotopes are used as tracers in medical imaging techniques such as PET and SPECT, allowing doctors to track the activity of bodily processes.
- Nuclear engineering: Understanding decay constants is essential for designing radiation protection systems in nuclear power plants and other facilities that handle radioactive materials.
By unraveling the relationship between decay constant and half-life, we gain a deeper understanding of the fundamental processes that govern the behavior of radioactive atoms. This knowledge empowers us to harness the applications of radioactivity in a safe and responsible manner.
Finding the Decay Constant Using Activity
In the world of nuclear physics, understanding the behavior of radioactive substances is crucial. One fundamental concept is the decay constant, which describes the rate at which a radioactive element decays. Determining the decay constant is essential for predicting the activity of a radioactive source and its applications in various fields.
Activity and Decay Constant
Activity, denoted by A, measures the number of radioactive decays occurring per unit time. It is directly proportional to the decay constant, λ. This relationship is expressed by the equation:
A = -λN
Here, N represents the number of radioactive atoms present in the sample. The negative sign indicates that activity is a decreasing quantity as radioactive atoms decay.
Significance of the Formula
The formula A = -λN signifies that the activity of a radioactive source is dependent on two crucial factors: the decay constant and the number of radioactive atoms present. By measuring the activity of a radioactive sample, scientists can infer the decay constant. This information is paramount in predicting the behavior of the substance over time.
Applications in Diverse Fields
Decay constants find widespread applications in various fields:
- Archaeology and Geology: Measuring the decay constant of radioactive isotopes like carbon-14 allows scientists to determine the age of fossils and geological formations through a technique known as radiocarbon dating.
- Medical Imaging: The decay constant of radioactive tracers used in medical imaging techniques, such as PET scans, helps determine the rate at which the tracer clears from the body. This information provides valuable insights for diagnostic and treatment purposes.
- Nuclear Engineering: In nuclear power plants, understanding the decay constants of nuclear fuels and radioactive waste is crucial for reactor safety and waste management. By accurately predicting the activity of radioactive materials, engineers can ensure proper shielding and containment measures.
Finding the Decay Constant Using Decay Rate
In the realm of radioactive decay, understanding the decay rate is crucial. Decay rate, commonly denoted by R, quantifies the fractional decay of radioactive atoms per unit time. It represents the rate at which atoms disintegrate and transform into a more stable form.
To derive the formula for decay rate, we start with the fundamental law of radioactive decay:
N(t) = N0e^(-λt)
Where:
– N(t) is the number of radioactive atoms at time t
– N0 is the initial number of radioactive atoms
– λ is the decay constant
Differentiating this equation with respect to time gives us:
dN(t)/dt = -λN0e^(-λt)
Simplifying further, we arrive at:
R = dN(t)/dt = -λN(t)
This equation establishes the relationship between decay rate (R) and decay constant (λ). It tells us that the decay rate is directly proportional to the number of radioactive atoms present at any given time.
In practice, the decay rate finds applications in various fields, such as nuclear engineering, medicine, and archaeology. By understanding the decay rate, scientists can accurately predict the behavior of radioactive materials and utilize them for various purposes.
Example:
In nuclear engineering, decay rate is used to calculate the shielding requirements for radioactive materials. The higher the decay rate, the more shielding is needed to protect workers and the environment from harmful radiation.
Understanding Exponential Decay
- Describe the exponential decay equation: N(t) = N0e^(-λt).
- Explain the role of initial atom count (N0) and elapsed time (t).
Understanding Exponential Decay
In the realm of nuclear physics, radioactive decay follows a fascinating pattern known as exponential decay. This pattern describes the gradual decrease in the number of radioactive atoms over time.
At the heart of exponential decay lies the exponential decay equation:
N(t) = N0e^(-λt)
where:
- N(t) represents the number of radioactive atoms remaining at time t
- N0 is the initial number of radioactive atoms at time t = 0
- λ is the decay constant, a measure of how quickly the atoms decay
Decoding the Exponential Decay Equation
The exponential decay equation unravels the relationship between the initial atom count and the elapsed time. The decay constant, λ, governs the rate at which radioactive atoms disintegrate. A larger decay constant indicates faster decay.
The Initial Atom Count (N0)
N0 signifies the starting point of radioactive decay. It represents the total number of radioactive atoms present at the outset. As time progresses, this number dwindles according to the exponential decay equation.
The Elapsed Time (t)
t symbolizes the duration since the start of radioactive decay. It is expressed in units of time, typically years or seconds. The elapsed time plays a crucial role in determining the rate of decay.
The Significance of Exponential Decay
Exponential decay finds applications in diverse fields, including:
- Archaeology and Geology: Carbon dating utilizes exponential decay to determine the age of ancient artifacts and geological formations.
- Medical Imaging: In radiology, radioactive isotopes undergo exponential decay, allowing for the visualization of internal organs and tissues.
- Nuclear Engineering: Decay constants guide radiation protection measures in nuclear power plants and other facilities handling radioactive materials.
By understanding exponential decay, scientists and professionals can harness its principles to unravel mysteries, advance medical diagnostics, and ensure safe handling of radioactive substances.
Finding the Decay Constant: A Comprehensive Guide
Radioactive decay is a fascinating phenomenon that governs the behavior of unstable atomic nuclei. Understanding this process is crucial in fields ranging from nuclear engineering to archaeology. Let’s delve into the concept of radioactive decay and explore how we can determine its key parameter, the decay constant.
Understanding Radioactive Decay
When an atomic nucleus is unstable, it undergoes radioactive decay to achieve a more stable state. This decay process can manifest in various forms, emitting radiation as particles or energy. Radioactive decay follows an exponential pattern, meaning the number of decaying atoms decreases exponentially over time.
Determining the Decay Constant
The decay constant (λ) is a critical parameter that characterizes the rate of radioactive decay. It represents the fraction of atoms decaying per unit time. There are several methods to determine the decay constant:
Using Half-life
Half-life (t½) is the time it takes for half of the radioactive atoms in a sample to decay. There is an inverse relationship between half-life and decay constant, expressed by the formula:
λ = ln(2) / t½
Using Activity
Activity (A) measures the rate of decay in a sample and is directly proportional to the decay constant. The formula is:
A = -λN
where N is the number of radioactive atoms.
Using Decay Rate
Decay rate (R) represents the fractional decay per unit time. The formula for decay rate is:
R = -λN
Exponential Decay
Radioactive decay follows an exponential decay pattern, described by the following equation:
N(t) = N0e^(-λt)
where:
- N(t) is the number of radioactive atoms at time t
- N0 is the initial number of radioactive atoms
- λ is the decay constant
- t is the elapsed time
This equation illustrates how the number of atoms exponentially decreases with increasing time.
Applications of Decay Constants
Decay constants find practical applications in diverse fields:
-
Carbon Dating (Archaeology and Geology): By measuring the ratio of different carbon isotopes, scientists use decay constants to determine the age of organic remains.
-
Medical Imaging (Radiology): Decay constants are used to calculate doses of radioactive tracers injected into patients, enabling non-invasive diagnosis and monitoring.
-
Nuclear Engineering (Radiation Protection): Engineers use decay constants to design shielding and safety measures for nuclear reactors and radioactive waste storage facilities.
Understanding decay constants is essential for comprehending radioactive processes and their applications across various scientific disciplines. By exploring the methods to determine decay constants, we gain valuable insights into the behavior of radioactive materials and their impact on our world.