A Comprehensive Guide To Covalent Bonding: Electron Sharing, Lewis Structures, And More
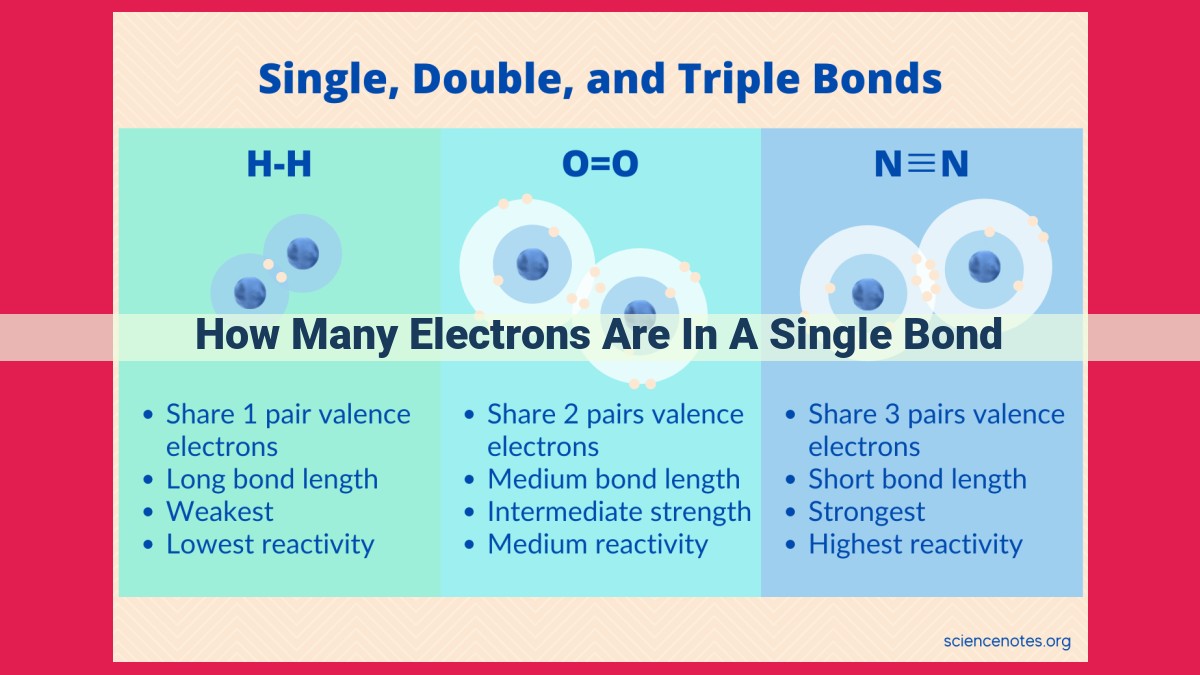
In a single bond, two atoms share two electrons to form a covalent bond. Lewis structures, which depict chemical bonding with dots representing valence electrons, provide a visual representation of this electron sharing. Electronegativity influences electron distribution, creating polar or nonpolar bonds. Bond length varies based on atomic radii and hybridization, while bond strength, determined by bond order and electron attraction, indicates the stability of the bond and the energy required to break it.
Delving into the World of Lewis Structures: A Journey of Chemical Bonding Explained
In the realm of chemistry, the enigmatic dance of atoms intertwining, forming the very foundation of matter, has long captivated scientists. At the heart of this intricate choreography lies a powerful tool known as Lewis structures. These graphical representations, named after their inventor, Gilbert Lewis, provide an elegant glimpse into the hidden forces that govern chemical bonding.
Lewis structures are essentially molecular blueprints, depicting the arrangement of atoms and their valence electrons. Valence electrons, the electrons residing in the outermost energy level of an atom, play a pivotal role in determining its bonding behavior. They are the driving force behind the attraction and repulsion between atoms, ultimately shaping the molecular architecture.
By understanding the concept of Lewis structures, we gain the ability to visualize the intricate web of chemical bonds that hold molecules together. We can unravel the secrets of molecular geometry, bond lengths, and bond strengths—properties that dictate the behavior and reactivity of substances. It’s a journey into the microscopic world where the interplay of atoms reveals the fundamental principles governing the material world around us.
The Significance of Valence Electrons: Unveiling the Secrets of Chemical Bonding
Imagine a grand dance, where atoms gracefully interact, exchanging electrons to create intricate patterns of bonds. Valence electrons, like skilled dancers, play a pivotal role in this cosmic choreography, determining the very nature of these chemical bonds.
Each atom possesses a unique number of valence electrons, those that reside in its outermost energy level. These electrons are eager to mingle, seeking out partners to form stable and satisfying bonds. When atoms have a surplus or deficit of valence electrons, they eagerly interact, forming bonds to achieve a blissful balance.
Covalent bonds arise when atoms share their valence electrons, creating a shared electron cloud that envelops both nuclei. This electron-sharing dance creates a strong electrostatic attraction between the atoms, binding them together. The number of shared electron pairs determines the bond order, an indicator of bond strength. Double and triple bonds, with their increased number of shared electron pairs, are significantly stronger than single bonds.
In the realm of chemical bonding, electronegativity also plays a significant role. This property, which measures an atom’s ability to attract shared electrons, influences the distribution of electrons within a bond. When atoms differ significantly in electronegativity, the resulting bond becomes polar, with electrons gravitating towards the more electronegative atom. This polarity can lead to the formation of ionic bonds, where one atom completely transfers an electron to another.
The dance of valence electrons not only determines the type of bond formed but also profoundly affects bond length and strength. Shorter bonds, with a smaller distance between the nuclei, are typically stronger than longer bonds. Hybridization, the mixing of atomic orbitals, can influence bond length and geometry, giving rise to diverse molecular shapes.
In conclusion, valence electrons are the key players in the intricate world of chemical bonding. Their dance dictates the strength, length, and polarity of bonds, shaping the molecular architecture of our universe. Understanding the role of valence electrons is essential for deciphering the secrets of chemical bonding and unlocking the mysteries of matter.
Electronegativity and Its Impact on Bond Properties
In the realm of chemistry, understanding the interactions between atoms is crucial. One fundamental concept that governs these interactions is electronegativity. Electronegativity measures an atom’s ability to attract and hold electrons in a chemical bond. This property plays a pivotal role in determining the distribution of electrons within a bond, influencing its polarity and strength.
The Concept of Electronegativity
Imagine a tug-of-war between two atoms sharing electrons in a bond. The one with a stronger desire to possess these electrons will exert a greater pull. This tug-of-war is influenced by the electronegativity of each atom. An atom with a higher electronegativity will have a stronger attraction for electrons, while an atom with a lower electronegativity will be less likely to hold onto them. This difference in electronegativity can lead to unequal sharing of electrons.
Polarity and Bond Strength
The unequal distribution of electrons in a bond results in polarity. Bonds between very different elements, where one atom avidly snatches electrons, can become highly polar. On the other hand, bonds between similar elements, where electrons are shared almost evenly, are considered nonpolar. The polarity of a bond affects its strength.
Polar bonds have regions of partial positive and partial negative charges, which can attract each other. This electrostatic attraction contributes to the overall bond strength. In contrast, nonpolar bonds have no such electrostatic attraction, making them generally weaker.
Examples in Action
To illustrate the impact of electronegativity, let’s consider the differences between two bonds:
-
Hydrogen-Fluorine (H-F): Fluorine is highly electronegative, so it strongly attracts electrons. The electrons in the H-F bond are much closer to fluorine than to hydrogen, resulting in a highly polar bond. This polarity makes the H-F bond relatively strong.
-
Carbon-Carbon (C-C): Both carbon atoms have similar electronegativities, resulting in an almost even distribution of electrons. Therefore, the C-C bond is nonpolar and generally weaker than the H-F bond.
By understanding electronegativity and its influence on bond properties, chemists can gain insights into the formation and behavior of chemical substances. This knowledge is essential for predicting molecular properties, designing materials, and comprehending various chemical phenomena.
Bond Length and Its Determinants
Delving into the Realm of Chemical Bonds
The world of chemistry is an intricate tapestry woven together by chemical bonds, which hold atoms together to form molecules. Understanding these bonds is crucial for unraveling the mysteries of the microscopic realm. Among the fundamental aspects of chemical bonds is their length, a key determinant of their strength and properties.
Atomic Radii: The Size Matters
Imagine atoms as tiny spheres, with their atomic radii dictating their size. Larger atoms, like giants, have more space between their outermost electrons and the nucleus, while smaller atoms, like diminutive elves, have their electrons closer to the core. This difference in atomic size directly influences bond length. When two large atoms form a bond, the distance between their nuclei is greater, resulting in a longer bond. In contrast, when two small atoms join forces, their nuclei are closer together, creating a shorter bond.
Hybridization: A Dance of Orbitals
The shape of an atom’s outermost orbitals, known as hybridization, also plays a pivotal role in bond length. Hybridization occurs when atomic orbitals combine to form new hybrid orbitals with unique shapes. Different types of hybridization, such as sp3, sp2, and sp, dictate the angles between the bonds, which in turn affects bond length. For instance, sp3 hybridization, with its tetrahedral shape, results in bonds that are directed away from each other, maximizing bond length.
Bonding Overlap: The Intimate Embrace
The extent to which atomic orbitals overlap during bond formation is another crucial factor in determining bond length. The greater the overlap, the stronger the bond and the shorter the bond length. Imagine two interlocking puzzle pieces; the more they overlap, the tighter the fit and the shorter the overall length. Similarly, in chemical bonds, the greater the orbital overlap, the closer the atoms are drawn together, resulting in a shorter bond length.
By unraveling the intricacies of bond length and its determinants, we gain a deeper understanding of the molecular world that surrounds us. From the humble formation of simple molecules to the complex interactions within living organisms, bond length plays a vital role in shaping the structure and properties of matter.
Bond Strength: A Tale of Attraction and Bond Integrity
In the symphony of chemical bonds, strength plays a crucial role, dictating the stability and resilience of molecules. Bond strength measures the force that holds atoms together, a testament to the dance of electrons that dictate their chemical union.
Bond strength is a captivating interplay of three key factors:
Bond Order: The Dance of Electron Pairs
The number of shared electron pairs between atoms determines the bond order. A single bond has one pair, a double bond two pairs, and so on. The higher the bond order, the stronger the bond. This is because more shared electrons mean a greater attraction between the atoms.
Bond Length: A Measure of Atomic Embrace
Bond length refers to the distance between the nuclei of bonded atoms. The shorter the bond length, the stronger the bond. This is because shorter distances allow for greater overlap of atomic orbitals, leading to a more effective sharing of electrons.
Strength of Electron Attraction: The Power of Electronegativity
Electronegativity measures an atom’s ability to attract electrons. When atoms with different electronegativities bond, the shared electrons are drawn closer to the more electronegative atom. This creates a polar bond with a partial positive charge on the less electronegative atom and a partial negative charge on the more electronegative atom. Polar bonds are generally weaker than nonpolar bonds where electrons are shared equally.
Bond Strength’s Significance: A Story of Molecular Stability and Reactivity
Bond strength has profound implications for the chemistry of molecules:
- High bond strength promotes molecular stability, allowing molecules to withstand external forces without breaking apart.
- Low bond strength makes molecules more reactive, as the bonds can be readily broken to form new chemical species.
Understanding bond strength is essential for unraveling the intricate chemical tapestry of the world around us. It’s a story of attraction, bonding, and the delicate balance that governs the formation and reactivity of molecules that shape our universe.