Understanding Covalent Bond Formation: Nucleotides And Phosphodiester Bonds
Covalent bond formation in nucleotides occurs via dehydration synthesis where water molecules are removed between the phosphate group of one nucleotide and the sugar of another. This results in the formation of a phosphodiester bond, a specific covalent bond that connects nucleotides to create polymers like DNA and RNA. The covalent nature of the phosphodiester bond involves the sharing of electron pairs, ensuring the bond’s strength and stability.
Dehydration Synthesis and Polymerization
- Describe dehydration synthesis as a chemical reaction that removes water molecules to form covalent bonds between monomers.
- Explain how polymerization involves multiple dehydration synthesis reactions to create polymers.
Understanding Dehydration Synthesis: The Key to Polymer Formation
Imagine a world where tiny building blocks called monomers combine to create larger, more complex structures known as polymers. This magical transformation is made possible by a chemical reaction called dehydration synthesis.
What is Dehydration Synthesis?
Dehydration synthesis is a process that involves removing water molecules from monomers to form covalent bonds between them. These covalent bonds are strong and stable, sharing electron pairs between atoms, creating a sturdy framework for polymers.
A Polymerizing Adventure
When multiple monomers undergo dehydration synthesis, the result is a polymer. Think of it like a chain reaction where one bond leads to another, forming a growing polymer backbone. Each addition of a monomer is like adding another link to the chain, creating a larger and more complex structure.
Unveiling the Role of Covalent Bonds
Covalent bonds are the foundation of polymers. They are formed when atoms share electrons, resulting in a stable and lasting connection. These bonds hold the polymer backbone together, giving it strength and shape.
Nucleotide Bonding: A Dehydration Synthesis Masterclass
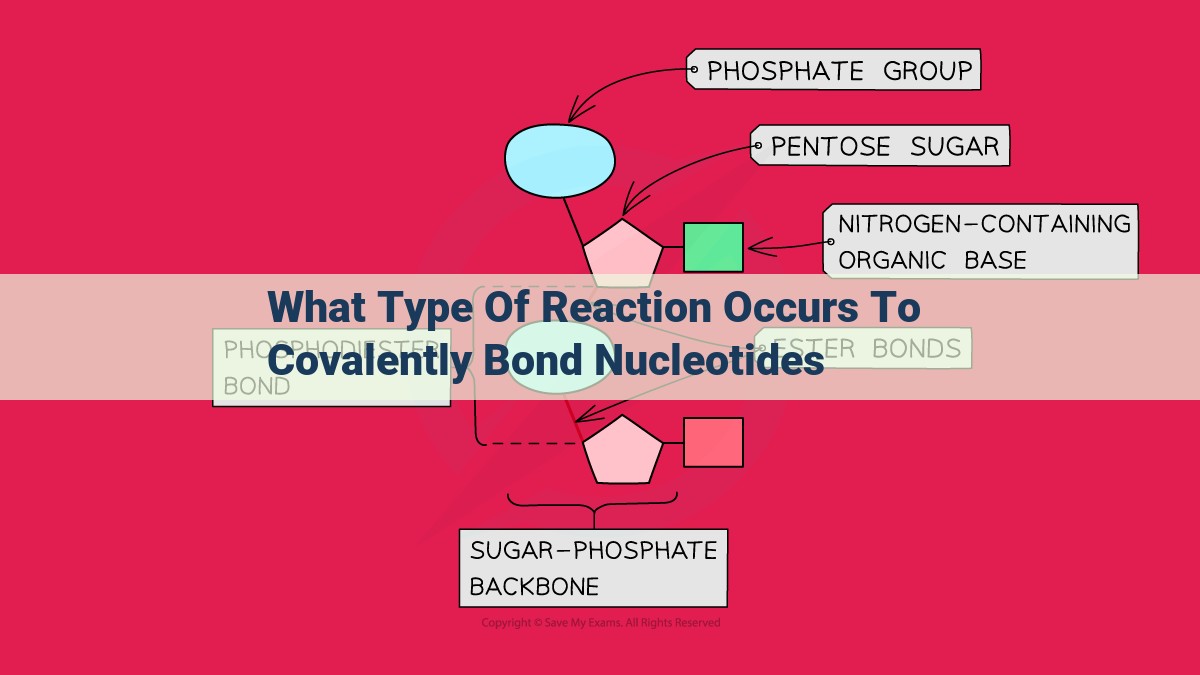
Nucleotides, the building blocks of DNA and RNA, are a prime example of dehydration synthesis in action. Each nucleotide consists of a nitrogenous base, a sugar, and a phosphate group.
The phosphate group of one nucleotide forms a phosphodiester bond with the sugar of another nucleotide. This covalent bond, created through dehydration synthesis, links nucleotides together, forming the polymeric backbone that makes up nucleic acids.
Dehydration synthesis and covalent bonds are essential concepts that underpin the structure and function of biological molecules. Understanding these processes is critical for unlocking the secrets of life’s building blocks and appreciating the intricate tapestry of life.
Delving into the Building Blocks of Life: Nucleotides
Life, in its intricate complexity, is built upon a foundation of molecules, and among these molecular players, nucleotides stand out as the building blocks of nucleic acids, the informational powerhouses of our cells.
The Essence of Nucleotides
Nucleotides, the very essence of genetic blueprints, are organic molecules composed of three fundamental components:
- Nitrogenous Base: A molecule derived from nitrogen and carbon, responsible for carrying genetic information.
- Sugar: A carbohydrate molecule, either ribose (RNA) or deoxyribose (DNA).
- Phosphate Group: A negatively charged molecule comprising a phosphorus atom bonded to oxygen atoms.
Covalent Bonding: The Unifying Force
The backbone ofnucleic acids, DNA and RNA, is formed by a chain of nucleotides linked together by covalent bonds, strong chemical bonds formed when atoms share electrons.
Phosphodiester Bonds: The Architectural Link
Specifically, the covalent bonds that connect nucleotides are called phosphodiester bonds, forged through a process known as dehydration synthesis. In this reaction, a phosphate group from one nucleotide reacts with a sugar molecule from another, releasing a water molecule and forming a bond.
These phosphodiester bonds are the molecular glue that holds nucleotides together, forming the polymeric backbones of DNA and RNA. These polymers, long chains of information, carry the genetic code that shapes and directs the functions of living organisms.
Covalent Bonds: The Unsung Heroes of Life’s Building Blocks
Imagine a world where everything was just a bunch of disconnected atoms, floating around aimlessly. Not very exciting, right? That’s where covalent bonds come in, the magical glue that binds atoms together and creates all the amazing stuff we know and love.
Covalent bonds are special types of chemical bonds where atoms share their electrons, creating a strong and stable connection. Picture this: two atoms, each with their own electrons, get cozy and start mingling. They decide to share their electrons, like two friends sharing a blanket. This shared ownership binds them together, creating a covalent bond.
This electron-sharing party has two main consequences. Firstly, it makes the atoms more chemically stable, like a team of superheroes joining forces to become invincible. Secondly, it creates a bond between the atoms that’s not as easily broken as other types of bonds. It’s like the atoms are holding hands, refusing to let go.
These covalent bonds are the foundation of everything in the world around us, from the air we breathe to the water we drink. They’re responsible for the structure of molecules, the building blocks of life. In fact, DNA, the blueprint for all living things, is held together by covalent bonds. Pretty amazing, right?
Phosphodiester Bond Formation: The Backbone of Genetic Information
In the intricate world of molecular biology, the formation of phosphodiester bonds plays a pivotal role in the very blueprint of life. These covalent linkages form the solid foundation of DNA and RNA, the molecules that encode our genetic heritage.
Dehydration Synthesis: The Building Blocks of Polymers
To understand phosphodiester bond formation, we must delve into the concept of dehydration synthesis. This chemical reaction is the driving force behind the assembly of many biological molecules, including DNA and RNA. In dehydration synthesis, water molecules are removed, allowing two molecules to form a covalent bond.
Nucleotide Structure: The Foundation of Genetic Code
At the heart of DNA and RNA lie nucleotides, the fundamental units of these genetic polymers. Each nucleotide consists of three components: a nitrogenous base, a sugar molecule, and a phosphate group. The nitrogenous base can be one of four different types: adenine (A), thymine (T), guanine (G), or cytosine (C). These bases pair up in specific combinations to create the genetic code.
Phosphate Group: The Key to Covalent Bonding
The phosphate group of a nucleotide holds the key to phosphodiester bond formation. When two nucleotides are aligned in a specific orientation, the phosphate group of one nucleotide reacts with the hydroxyl group on the sugar of the other nucleotide. This dehydration synthesis reaction releases a water molecule and forms a covalent bond known as a phosphodiester bond.
Phosphodiester Bonds: The Structural Backbone of Genetic Molecules
The resulting phosphodiester bond is an incredibly stable linkage that connects the nucleotides together. As multiple dehydration synthesis reactions occur, a growing chain of nucleotides forms, creating the polymeric backbone of DNA or RNA. This backbone provides the structural framework for the genetic code, allowing for the precise storage and transmission of hereditary information.
In summary, phosphodiester bond formation is the fundamental process that gives rise to DNA and RNA, the molecules that carry the blueprint of life. Through dehydration synthesis, nucleotides are linked together by stable covalent bonds, forming the backbone of these essential biological polymers. Understanding this process is crucial for unraveling the secrets of genetics and the intricacies of cellular machinery.