Core Electrons: Key Players In Atomic Structure And Chemical Behavior
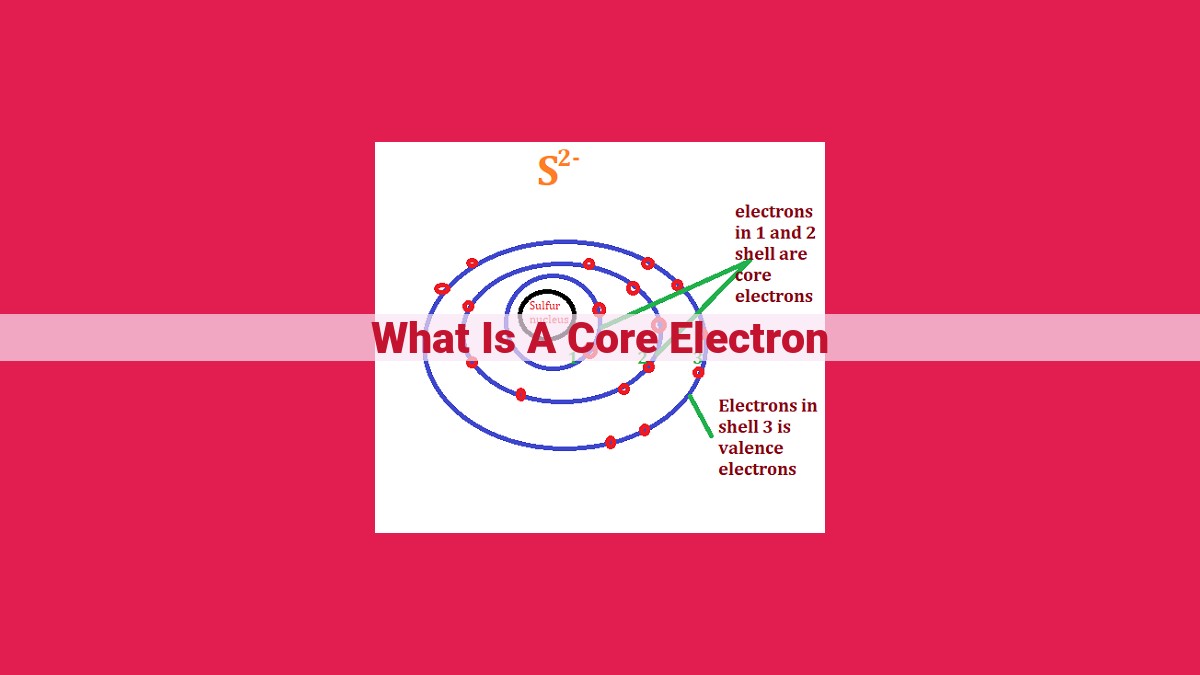
Core electrons are tightly bound to the nucleus, occupying the innermost atomic orbitals. They strongly influence an atom’s chemical properties and do not participate in chemical bonding. Their existence contributes to the periodic table’s organization and helps explain various atomic phenomena.
- Definition and importance of core electrons
- Explain their role in atomic structure and chemical properties
Core Electrons: The Foundation of Atomic Chemistry
In the realm of chemistry, where atoms dance and interact to create the world around us, there’s a hidden layer within each atom that holds the key to its structure and behavior: core electrons.
Defining Core Electrons
Core electrons are the innermost electrons in an atom, positioned closest to the nucleus, the heart of the atom. These electrons are tightly bound to the positively charged nucleus by a strong electromagnetic force. Their proximity to the nucleus makes them crucial to the atom’s stability.
Role in Atomic Structure
Core electrons play a vital role in determining an atom’s overall shape, size, and energy levels. They occupy the lowest energy orbitals, providing a foundation for the building blocks of the atom. By shielding the nucleus from the outer electrons, they help stabilize the electrons’ movement and contribute to the atom’s overall electron configuration.
Influence on Chemical Properties
The properties of an atom’s core electrons heavily influence its chemical behavior. Because core electrons are tightly bound to the nucleus, they are not readily available for chemical reactions. This inertness makes them less reactive compared to outer electrons, which are involved in chemical bonding and determine an atom’s chemical properties.
Core Electrons: Properties
Nestled deep within the heart of an atom, you’ll find the core electrons. These fundamental particles reside in the innermost atomic orbitals, closest to the nucleus. Their location and proximity to the nucleus grant them a special status within the atomic realm.
Core electrons are bound to the nucleus with exceptional strength. The nucleus, with its positively charged protons, exerts a strong electrostatic force that draws the negatively charged electrons towards it. This bond is so powerful that it prevents the core electrons from straying far from their designated orbits.
Unlike their more adventurous counterparts, the valence electrons, core electrons don’t actively participate in chemical bonding. They remain steadfast in their orbits, content to maintain the atom’s stability. This unwavering nature is what sets them apart from valence electrons, which eagerly engage in bonding adventures.
Their immobility doesn’t diminish their importance. Core electrons play a crucial role in determining the **chemical properties of an element.** They influence the atom’s size, ionization energy, and electron affinity, all of which shape its reactivity and behavior in chemical reactions.
Understanding core electrons is essential for grasping the intricacies of atomic chemistry. They form the foundation upon which the chemical properties of elements are built. Without them, the dance of electrons that drives chemical reactions would lack the stability and predictability that makes it possible.
Related Concepts: Atomic Orbital and Electron Configuration
- Describe atomic orbitals and their role in electron distribution
- Explain electron configuration as the arrangement of electrons in orbitals
Atomic Orbitals and Electron Configuration: Delving into the Quantum Realm
As we delve into the intricate world of atoms, it’s essential to understand atomic orbitals—the fundamental building blocks of electron distribution. These orbitals are three-dimensional regions around the nucleus where electrons are most likely to be found. Each orbital represents a specific energy level, with the s orbital being the lowest energy and closest to the nucleus, followed by p, d, and f orbitals in increasing energy.
The electron configuration of an atom is the arrangement of its electrons in these atomic orbitals. Each electron occupies an orbital in accordance with its energy and other quantum properties. The electron configuration of an element determines its chemical properties, as it dictates the number of valence electrons—the electrons in the outermost energy level that are available for chemical bonding.
Understanding atomic orbitals and electron configuration is crucial for comprehending the behavior of electrons in atoms. These concepts provide a framework for understanding the structure and properties of elements and the formation of chemical compounds. By delving into these quantum realms, we unlock a deeper understanding of the fundamental building blocks of matter and the intricate dance of electrons within them.
The Tale of Valence Electrons: The Building Blocks of Chemistry
In the vast realm of atoms and their intriguing world, we encounter the unsung heroes known as valence electrons. Residing in the outermost energy levels of atoms, these electrons hold the key to unraveling the secrets of chemical reactivity and bonding. Valence electrons are like the sociable extroverts of the atomic world, eager to interact with electrons from other atoms.
These gregarious electrons play a pivotal role in shaping an atom’s chemical personality. They determine an atom’s willingness to participate in chemical bonding, which is the dance of atoms coming together to form molecules. The dance is driven by the desire of valence electrons to achieve a stable configuration, a harmonious state where they neither give up nor receive extra electrons.
The Importance of Valence Electrons
The number of valence electrons an atom possesses is a guiding star in predicting its chemical behavior. Elements with the same valence electron count tend to exhibit similar chemical properties. For instance, the alkali metals (Group 1 on the Periodic Table) all have one valence electron, which explains their characteristic reactivity and tendency to lose that electron to form cations.
The ability of valence electrons to engage in chemical bonding is fundamental to the formation of compounds, the building blocks of our world. From the oxygen we breathe to the polymers that make up our clothes, chemical bonding enabled by valence electrons is the driving force behind the existence of matter as we know it.
In conclusion, valence electrons are the gatekeepers of chemical reactivity, dictating the nature of the dance between atoms. Understanding the role of valence electrons is a stepping stone toward unraveling the enchanting tapestry of chemistry.
Atomic Anatomy: Core Electrons, Their Influence, and Beyond
In the realm of chemistry, unraveling the mysteries of atoms is akin to embarking on an extraordinary exploration. Core electrons, the unsung heroes at the heart of this microscopic world, play a pivotal role in defining atomic identity and governing chemical behavior.
The atomic number, a fundamental characteristic of each element, represents the number of protons lurking within the nucleus, the atom’s central command center. Its spiritual companion, the mass number, embodies the combined forces of protons and neutrons, the heavyweights of the nucleus. These numerical signatures act as fingerprints, distinguishing one element from another and giving rise to the enchanting diversity of the periodic table.
Atomic number and mass number, the cornerstones of atomic identity, unveil the secrets of isotopes. These siblings, sharing the same atomic number but differing in mass number, bear a striking resemblance yet possess subtly distinct personalities. Isotopes arise from variations in the neutron count, influencing the overall mass of the atom without altering its elemental nature.
To complete our atomic narrative, let us meet the valence electrons, the social butterflies of the atomic world. Residing in the outermost energy levels, they dance freely, venturing into chemical interactions that determine an atom’s bonding prowess and reactivity. These extroverted electrons hold the key to understanding the formation of molecules, the building blocks of our material universe.
Thus, the tapestry of atomic chemistry is woven together by a symphony of intertwined concepts: core electrons, atomic number, mass number, isotopes, and valence electrons. Each thread plays a unique role in shaping atomic properties and orchestrating chemical reactions. Embracing these fundamental building blocks empowers us to decipher the complexities of the atomic world and unravel the intricate dance of chemical transformations.
Isotopes: Atoms with a Twist
In the realm of chemistry, we encounter elements, the building blocks of all matter. Each element is characterized by its unique atomic number, which represents the number of protons within its nucleus. However, nature has a clever trick up its sleeve: isotopes. Isotopes are atoms of the same element that share the same atomic number but differ in their mass number.
The mass number of an atom is the total number of protons and neutrons found within its nucleus. While the number of protons remains constant for a given element, the number of neutrons can vary. This variation in neutron count gives rise to different isotopes of the same element.
For example, carbon, an element crucial for life on Earth, has three naturally occurring isotopes: carbon-12, carbon-13, and carbon-14. All three isotopes have six protons in their nuclei, but they differ in their neutron count. Carbon-12 has six neutrons, carbon-13 has seven neutrons, and carbon-14 has eight neutrons.
These variations in neutron count have subtle but important implications. Isotopes with different neutron numbers possess slightly different physical and chemical properties. Carbon-12, for instance, is the most abundant isotope of carbon, while carbon-14 is a radioactive isotope used in radiocarbon dating.
Isotopes play a vital role in various scientific fields. They are utilized in medical imaging, environmental studies, and even nuclear power generation. Understanding isotopes and their properties is essential for advancements in these fields and for deepening our knowledge of the fundamental nature of matter.
Ions: The Charged Entities of Chemical Reactions
In the realm of chemistry, atoms, the fundamental building blocks of matter, undergo fascinating transformations to form ions. These are electrically charged particles that play a pivotal role in countless chemical reactions. Understanding ions expands our comprehension of atomic structure and their behavior in chemical processes.
The Creation of Ions: A Balancing Act
Ions emerge when atoms or molecules lose or gain electrons, the negatively charged particles that orbit the nucleus. When an atom loses one or more electrons, it becomes positively charged and is known as a cation. Conversely, when an atom gains electrons, it acquires a negative charge and becomes an anion.
Cations and Anions: A Tale of Electron Exchange
The formation of cations and anions stems from the attraction between oppositely charged particles. When an atom loses electrons, it exposes its positively charged protons, resulting in a cation with a net positive charge. In contrast, when an atom gains electrons, it acquires a net negative charge, forming an anion.
For example, sodium (Na), a highly reactive metal, readily loses one electron, becoming a sodium cation (Na+) with a positive charge. Chlorine (Cl), a greenish-yellow gas, eagerly accepts an electron, transforming into a chloride anion (Cl-) with a negative charge.
The Significance of Ions in Chemical Reactions
Ions play a crucial role in numerous chemical reactions, including the formation of ionic compounds, the conduction of electricity in solutions, and the regulation of physiological processes in living organisms. Their ability to interact through electrostatic forces enables the creation of stable chemical bonds, leading to the formation of ionic compounds.
Furthermore, ions serve as charge carriers in solutions, allowing the flow of electricity. This property has immense applications in batteries, electroplating, and other electrochemical processes. In the human body, ions such as sodium, potassium, and calcium are essential for maintaining proper nerve function, muscle contractions, and fluid balance.
By delving into the world of ions, we gain a deeper understanding of the intricate interactions between atoms and molecules, unlocking the secrets of chemical transformations and the workings of our natural world.