Copper Density: Understanding Mass-To-Volume Ratio And Its Importance
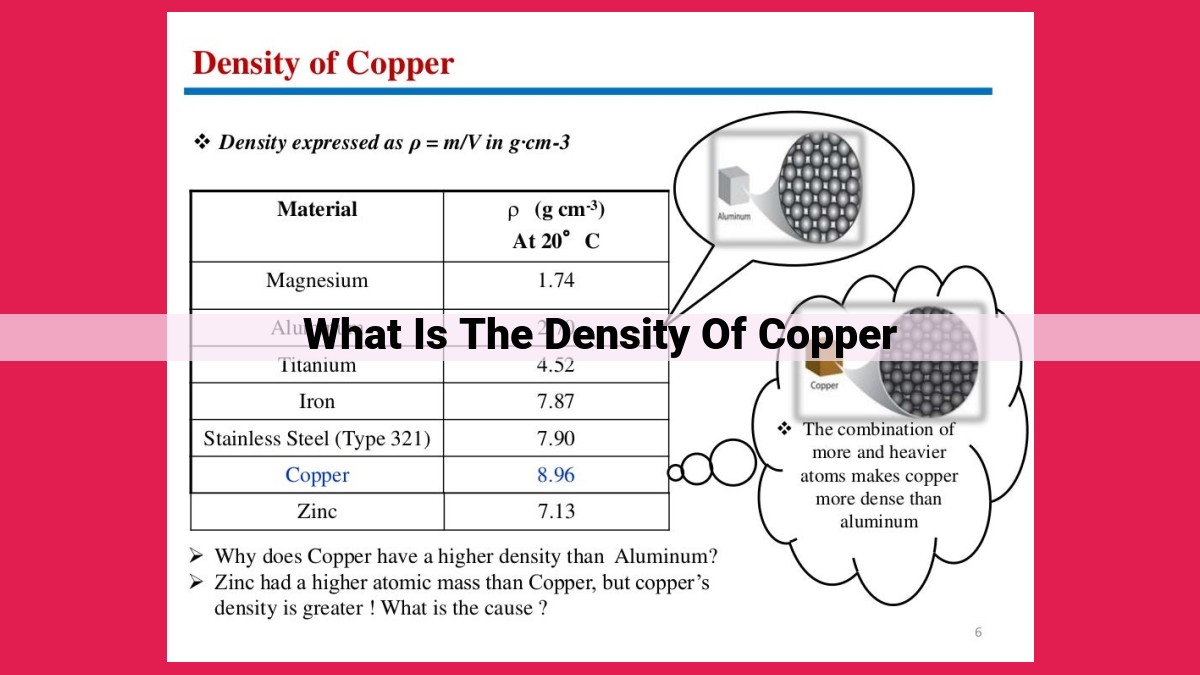
The density of copper is a measure of its mass per unit volume. It is an important property in understanding the characteristics of copper and is used in various applications. The density of copper can be calculated by dividing the mass of a copper sample by its volume. Common units for density are grams per cubic centimeter (g/cm³). Factors such as temperature, pressure, composition, and impurities can influence the density of copper. Understanding density is essential for purity testing, buoyancy analysis, and various engineering and scientific applications where knowledge of a material’s mass-to-volume ratio is crucial.
Understanding Density: The Cornerstone of Identifying Substances
In the vast repertoire of physical properties that define the identity and behavior of substances, density stands out as a fundamental pillar. It plays a pivotal role in characterizing substances, allowing us to differentiate between them with precision. Density serves as a constant companion, accompanying us in our everyday encounters and in the complex scientific investigations that unravel the secrets of the universe. Embark on this enlightening journey as we explore the multifaceted nature of density and uncover its significance in our world.
Defining Density: A Measure of Compactness
Density, denoted by the Greek letter rho (ρ), quantifies the compactness of a substance. It is defined as the ratio of mass, the amount of matter in an object, to volume, the space it occupies. This simple yet profound concept reveals how much substance is packed into a given space. A substance with a higher density is said to be more tightly packed, while a substance with a lower density has more void space.
The Importance of Density in Characterizing Substances
The significance of density in characterizing substances cannot be overstated. It serves as a fundamental property, providing invaluable insights into the internal structure and properties of matter. By determining the density of a substance, we can gain crucial information about its composition, purity, and even its molecular arrangement.
Density plays a particularly important role in the field of chemistry. It allows us to distinguish between different substances and identify unknown compounds. For example, the density of gold is significantly higher than that of silver, allowing us to differentiate between these two precious metals with ease.
In the realm of geology, density is utilized to identify different types of rocks and minerals. Sedimentary rocks, formed from the accumulation of particles, typically have lower densities than igneous rocks, which originate from the cooling of molten magma.
Beyond the realm of physical sciences, density finds applications in diverse fields such as biology, medicine, and engineering. In medicine, density measurements are used to assess body composition and diagnose certain diseases. In engineering, density is a critical factor in designing structures, ensuring stability and preventing catastrophic failures.
Overall, density is a versatile and essential property that provides a wealth of information about the nature of matter. Its applications extend far and wide, making it an indispensable tool in both scientific research and everyday life.
Measuring Density: A Guide to Quantifying Matter’s Compactness
Understanding density is crucial in characterizing various substances. It provides insights into their physical properties and behavior. To determine the density of a material, scientists have developed precise measurement techniques using specialized instruments.
Units of Density
The most commonly used unit of density is kilograms per cubic meter (kg/m³). Other units include *grams per cubic centimeter (g/cm³) and pounds per cubic foot (lb/ft³). These units represent the mass of a substance per unit volume.
Formula for Density
The formula for calculating density is:
Density = Mass / Volume
where:
- Mass (m): Measured in kilograms (kg)
- Volume (V): Measured in cubic meters (m³)
Measurement Techniques
Measuring the mass of an object is relatively straightforward. It can be done using a balance or a scale.
Measuring the volume of a regular object, such as a rectangular block, requires measuring its length, width, and height. Irregularly shaped objects require more specialized techniques, such as fluid displacement. This involves submerging the object in a known volume of fluid and measuring the change in volume.
Case Study: Measuring the Density of Copper
As an example, let’s measure the density of copper.
- Measure the mass: Using a balance, we measure the mass of a copper sample as 50 grams.
- Measure the volume: We use fluid displacement and measure the change in volume of water as 10 cubic centimeters when the copper sample is submerged.
- Calculate density: Using the formula, we get: Density = 50 grams / 10 cubic centimeters = 5 grams per cubic centimeter.
Therefore, the density of the copper sample is 5 g/cm³.
Factors Influencing Density
Understanding the density of a substance is crucial in characterizing its properties and behavior. While density is typically presented as a constant value, it’s essential to recognize that certain factors can influence its value. Exploring these factors provides a deeper understanding of the dynamics of density.
Impact of Temperature on Density
Temperature plays a significant role in determining the density of a substance. Generally, as temperature increases, the density of a substance decreases. This is because the increased thermal energy causes the molecules to move more vigorously, resulting in an expansion of volume. For instance, the density of water decreases as it heats up, which is why hot water rises above cold water.
Effects of Pressure on Density
Pressure can also affect the density of a substance. In general, applying increased pressure on a substance increases its density. This occurs because the external pressure forces the molecules closer together, reducing the volume occupied by the substance. This principle is particularly relevant in the study of deep-sea environments, where the immense pressure at great depths significantly increases the density of water.
Influence of Composition and Impurities on Density
The composition of a substance and the presence of impurities can also impact its density. Different elements and compounds have varying densities, affecting the overall density of the mixture. For example, the density of an alloy made from two metals will typically fall between the densities of the individual metals. Impurities, on the other hand, can alter the density of a substance by introducing voids or irregularities, which can either increase or decrease its overall density.
Applications of Density
Understanding density unlocks a wealth of applications in diverse fields. Let’s explore some of its fascinating uses:
-
Purity Testing using Density Analysis: Density is a hallmark of substance identity. By meticulously measuring the density of a sample, experts can determine its purity. This technique is extensively used in laboratories to ensure the genuineness of products, ranging from pharmaceuticals to precious metals.
-
Buoyancy Principles and Flotation Devices: Density plays a crucial role in the principles of buoyancy. Submerse an object in a fluid, and the upward buoyant force it experiences will be equal to the weight of the fluid it displaces. This concept underpins the operation of flotation devices, such as boats and life jackets.
-
Practical Applications of Density in Engineering and Science: Density finds myriad uses in engineering and science. In civil engineering, accurate knowledge of soil density is essential for constructing stable foundations. Chemists rely on density measurements to calculate molar mass and determine the concentration of solutions. The list goes on, highlighting the versatility of density as a fundamental property.
Case Study: Measuring the Density of Copper
Let’s venture into a hands-on example: measuring the density of copper. Follow these steps to embark on this experiment:
-
Gather Equipment: You’ll need a precision balance, calipers, a graduated cylinder, and a copper sample.
-
Determine the Mass: Precisely measure the mass of the copper sample using the balance, recording it as ‘m’.
-
Measure the Volume: Submerge the copper sample in a graduated cylinder filled with water, noting the initial water level as ‘V1’ and the final level after submersion as ‘V2’. The difference (‘V2-V1’) gives you the volume of the sample.
-
Calculate Density: Use the formula: Density = Mass / Volume, plugging in the values ‘m’ for mass and (‘V2-V1’) for volume. This calculation reveals the density of your copper sample.
Experimenting with density measurement not only demonstrates its practical applications but also reinforces its theoretical foundation.
Case Study: Delving into the Density of Copper
In the realm of science, understanding the properties of materials is crucial. One fundamental property is density, which plays a significant role in characterizing substances. In this case study, we’ll embark on an experimental journey to determine the density of copper, a metal widely used in industries. Join us as we unravel the steps involved in this fascinating process.
Materials Required:
- Copper sample (regular or irregular shape)
- Analytical balance
- Graduated cylinder or volumetric flask
- Water
Step 1: Determining the Mass of Copper
Using an analytical balance, meticulously measure and record the mass of the copper sample in grams (g). Ensure accuracy by ensuring the balance is calibrated and zeroed before weighing.
Step 2: Measuring the Volume of Copper (Regular Shape)
If your copper sample has a regular shape (e.g., cube, sphere, cylinder), you can calculate its volume using geometric formulas. Measure the dimensions of the sample (length, width, height, or diameter) with precision and substitute these values into the appropriate formula.
Step 3: Measuring the Volume of Copper (Irregular Shape)
For irregular-shaped samples, we resort to the water displacement method. Fill a graduated cylinder or volumetric flask with water and record the initial volume in milliliters (mL). Carefully submerge the copper sample in the water, ensuring it doesn’t trap any air bubbles. The increase in water volume corresponds to the volume of the copper sample in mL.
Step 4: Calculating the Density of Copper
Now, we can determine the density of copper using the formula:
Density = Mass / Volume
Substitute the measured mass in g from Step 1 and the calculated volume in mL from Step 2 or 3 into this formula. The result will be the density of copper in g/mL.
Through this experimental case study, we have successfully measured the density of copper. This value is not only a fundamental characteristic of the metal but also has practical applications in various fields, such as purity testing and buoyancy studies. By following these steps with precision, you can confidently determine the density of materials in your future experiments.