Understanding Copper (Cu): Atomic Number, Properties, And Reactivity
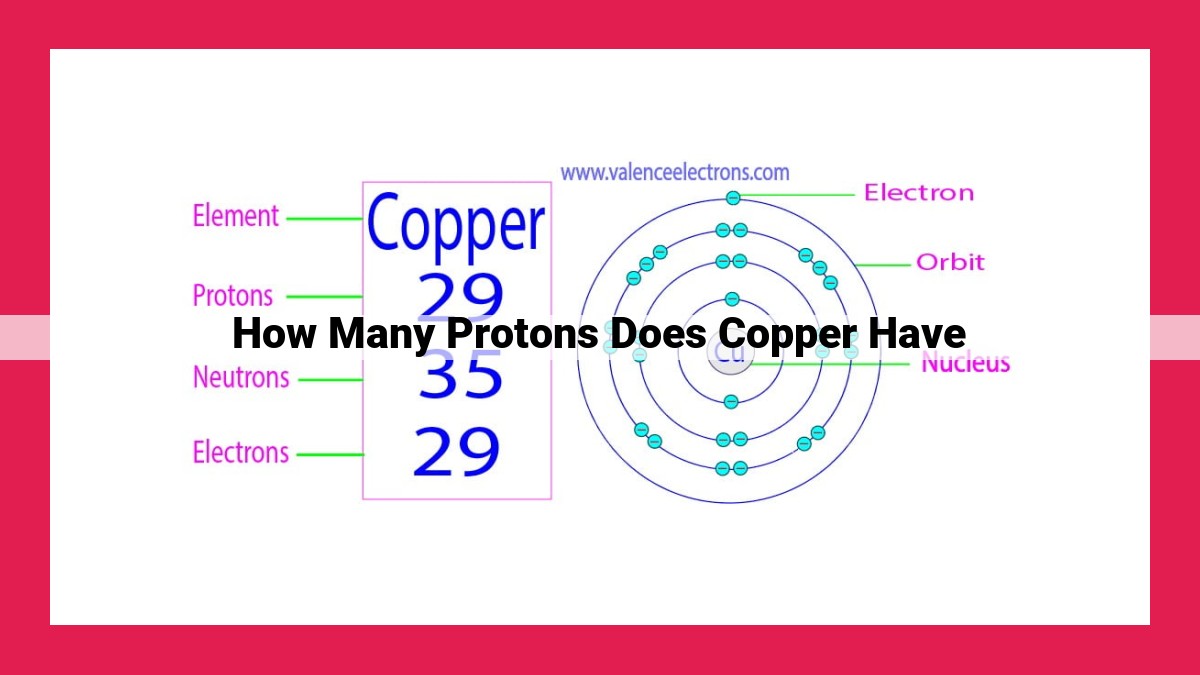
Copper’s atomic number, which determines the number of protons in its nucleus, is 29. This is represented by its element symbol, Cu. Protons carry a positive charge and contribute to the atom’s overall electromagnetic properties, shape, and reactivity.
Atomic Number
Definition: The number of protons in an atom’s nucleus
Related Concepts: Element symbol, mass number
Atomic Number: Unraveling the Core of Every Atom
In the vast and intricate tapestry of the universe, the building blocks of matter hold profound significance. Enter atomic number, an enigmatic concept that unravels the very heart of atoms.
An atomic number is the number of protons, positively charged particles that reside within an atom’s nucleus. This unique identifier determines the element to which an atom belongs. Each element in the periodic table has a distinctive atomic number, distinguishing it from all others.
For instance, copper, a metal renowned for its malleability and reddish-brown hue, bears atomic number 29. This implies that every copper atom harbors 29 protons in its nucleus. This fundamental property endows copper with its characteristic physical and chemical properties.
The atomic number is inextricably linked to other crucial atomic attributes. It fosters a harmonious relationship with the element symbol. This symbol, often a one- or two-letter abbreviation, represents the element and its atomic number. For copper, its element symbol, Cu, encapsulates its atomic number and unique identity.
Delving deeper, the atomic number also intertwines with the mass number. The mass number signifies the total number of protons and neutrons in an atom’s nucleus. While the atomic number determines an element’s identity, the mass number distinguishes different isotopes of the same element.
The atomic number serves as an invaluable tool for scientists, enabling them to understand the structure, properties, and interactions of atoms. It underpins the very foundation of chemistry and provides a guiding light in the exploration of the microscopic world.
Protons: The Guardians of Atomic Identity
In the vast expanse of the atomic realm, where tiny particles dance and determine the building blocks of our universe, protons stand as the steadfast sentinels of an atom’s identity. These subatomic particles, small yet mighty, hold the key to understanding the elements that make up our world.
Positive Pioneers
Protons, as their name suggests, possess a positive electrical charge, a fundamental force that shapes their role in the atomic orchestra. This charge creates an invisible barrier around the atom’s nucleus, the central core that houses protons and neutrons. Protons, like brave knights, guard the nucleus, repelling the advances of other positively charged particles.
The Atomic Gatekeepers
The number of protons in an atom’s nucleus is its atomic number, a crucial property that defines the element to which it belongs. Each element on the periodic table has a unique atomic number, which determines its chemical behavior and distinct characteristics. Copper, for instance, has an atomic number of 29, indicating that its nucleus harbors 29 protons.
The Positive Influence
The positive charge of protons has a profound influence on an atom’s structure and behavior. It attracts negatively charged electrons, creating a dynamic balance that holds the atom together. This electrostatic dance between protons and electrons gives atoms their stability and unique chemical properties.
Partners in Identity
Protons don’t work alone in defining an atom’s identity. They partner with element symbols, one- or two-letter abbreviations that represent each element. The element symbol for copper is Cu, a shorthand symbol that captures the essence of this 29-proton element.
Protons are the positive guardians of atomic identity, the gatekeepers that define the elements that shape our universe. Their positive charge and unique atomic numbers determine an atom’s chemical behavior and pave the way for the diverse properties of the elements that make up our world. Understanding protons is fundamental to unraveling the secrets of matter and appreciating the intricate dance of subatomic particles that drives our universe.
Element Symbol
Definition: A one- or two-letter abbreviation for a chemical element
Related Concepts: Atomic number, protons
Element Symbol: The Chemical Identifier
The world around us is a symphony of elements, the building blocks of matter. Each element possesses a unique identity, represented by its element symbol. These symbols, often consisting of one or two letters, are not mere placeholders but gateways to understanding the countless intricacies of the chemical universe.
An element’s symbol encapsulates its essence, providing crucial information about its atomic structure. Atomic number is the key to unraveling this identity. It represents the number of protons within an atom’s nucleus, the very core of its existence. Protons are subatomic particles carrying a positive charge, determining an element’s chemical properties and distinguishing it from other elements.
The element symbol serves as a constant companion to the atomic number, reflecting this fundamental characteristic. For instance, the element copper, with its atomic number of 29, proudly bears the symbol Cu. This enduring symbol not only identifies copper but also hints at its exceptional properties. Copper’s unique ability to conduct electricity and its warm, reddish-brown hue have made it an indispensable material throughout human history.
Element symbols are indispensable tools in the language of chemistry, enabling us to navigate the periodic table with ease. They simplify complex atomic structures, allowing for the swift communication of chemical information. These symbols are the shorthand of the chemical world, a testament to the power of human ingenuity in condensing the complexities of nature into concise and meaningful representations.
By embracing the element symbol, we unlock a deeper understanding of the elements that constitute our world. It is a gateway to exploring the vast realm of chemistry, a language that unravels the secrets of the universe one element at a time.
Copper’s Atomic Number: Unveiling the Essence of Copper
In the intricate tapestry of the atomic world, each element holds its own unique identity defined by its atomic number. This number, like a celestial address, reveals the fundamental essence of an element, dictating its properties and shaping its behavior. Let us embark on a journey to discover the atomic number of copper, an element renowned for its warmth, malleability, and enduring presence in human history.
Copper, with its atomic number of 29, occupies a distinct position on the periodic table. This number represents the very heart of copper’s atomic structure, the nucleus, where 29 positively charged subatomic particles known as protons reside. Like celestial bodies orbiting a star, these protons determine the element’s identity, distinguishing it from all others.
The atomic number of copper is not merely a numerical value; it is a window into the element’s properties and its place in chemistry. Copper’s unique character emerges from this atomic fingerprint, endowing it with a versatility that has captivated civilizations for millennia.
Beyond its atomic number, copper’s identity is further defined by its element symbol, Cu. This concise abbreviation serves as a shorthand for copper’s atomic individuality, linking it to the myriad compounds and alloys that have shaped human innovation.
Understanding copper’s atomic number not only provides a basis for comprehending its chemical behavior but also opens a door to unlocking its potential in various fields. From its electrical conductivity to its role in biological processes, copper’s unique atomic structure underlies its many applications.
In conclusion, the atomic number of copper, 29, stands as a testament to the power of atomic structure in defining an element’s essence. It is a cornerstone of copper’s unique character, paving the way for its widespread use in art, technology, and countless other facets of human endeavor.