Convert Moles To Millimoles: How Many Millimoles In A Mole?
How Many Millimoles in a Mole?
In chemistry, the mole and millimole are units used to measure the amount of a substance. A mole contains 6.022 x 10^23 particles (molecules, atoms, or ions), while a millimole is one-thousandth of a mole. To convert between moles and millimoles, the conversion factor of 1 millimole = 0.001 moles is used. For example, 3 moles of a substance would be equal to 3000 millimoles.
Understanding the Mole: A Unit of Measurement
- Explain the concept of the mole as a unit for measuring the amount of substance.
- Discuss related concepts such as millimole, molar mass, Avogadro’s number, and conversion factors.
Understanding the Mole: A Fundamental Unit in Chemistry
In the realm of chemistry, measuring the quantity of matter is crucial for deciphering the intricacies of chemical reactions. Among the many units of measurement designed for this purpose, the mole stands out as a cornerstone concept.
Imagine a massive assembly of countless tiny building blocks. The mole represents a specific number of these elementary units, similar to how a dozen represents a collection of twelve items. Just as a dozen eggs or a dozen pencils refers to a precise quantity, the mole denotes an exact amount of particles. In the case of chemistry, these fundamental building blocks are atoms, molecules, ions, or electrons.
The precise value of a mole is defined by a constant known as Avogadro’s number, which is an astonishingly large number: 6.022 × 10^23. This means that one mole contains 6.022 × 10^23 particles of the substance in question.
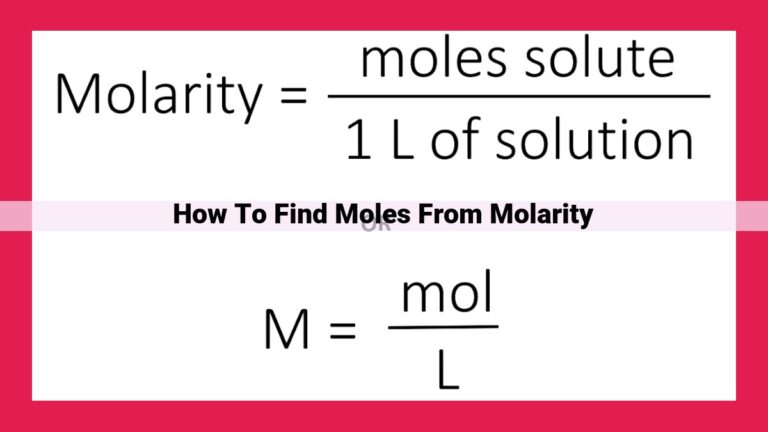
To facilitate conversions between the mole and smaller units, we utilize the millimole (mmol). One millimole is one-thousandth of a mole, just as one milliliter is one-thousandth of a liter. This conversion factor allows us to express quantities in either moles or millimoles as needed.
Another important concept closely related to the mole is molar mass. Molar mass represents the mass of one mole of a substance expressed in grams. It is essentially a measure of the “molecular weight” of a substance. By knowing the molar mass, we can convert between the mass and the number of moles in a sample.
Understanding the mole, millimole, and molar mass lays the foundation for quantitative analysis in chemistry. These units allow scientists to accurately measure and compare the amounts of reactants and products in chemical reactions, unraveling the secrets of matter and its transformations.
The Millimole: A Smaller Unit
In the world of chemistry, the mole reigns supreme as the unit for measuring the amount of substance. However, sometimes you need a smaller, more manageable unit, and that’s where the millimole (mmol) comes in.
The millimole is simply a fraction of a mole, just like a milligram is a fraction of a gram. One millimole is equal to one-thousandth of a mole (1 mmol = 0.001 mol). This makes it an ideal unit for measurements involving smaller quantities of substances.
The millimole and the mole are closely related, and you can easily convert between the two units using the conversion factor:
1 mmol = 0.001 mol
1 mol = 1000 mmol
For example, if you have 5 millimoles of a substance, you can convert it to moles by multiplying by 0.001:
5 mmol * 0.001 mol/mmol = 0.005 mol
Conversely, if you have 0.25 moles of a substance, you can convert it to millimoles by multiplying by 1000:
0.25 mol * 1000 mmol/mol = 250 mmol
Understanding the millimole is essential for accurate measurements and calculations in chemistry. By using the correct units and conversion factors, you can ensure the precision and reliability of your work.
Conversion Factor: Your Bridge Between Moles and Millimoles
In the realm of chemistry, understanding the relationship between moles and millimoles is crucial. Moles, as the SI unit for measuring the amount of substance, quantify the number of particles present. Millimoles, on the other hand, offer a smaller yet convenient unit, especially for quantitative analysis and smaller-scale reactions.
To seamlessly navigate between these units, the conversion factor serves as our bridge. Defined as 1 mole = 1000 millimoles, this factor allows us to effortlessly convert between moles and millimoles.
Let’s dive into an example to illustrate its practical application. Suppose you have a sample containing 0.2 moles of a substance and you need to express it in millimoles. Using the conversion factor, we can calculate:
0.2 moles * (1000 millimoles / 1 mole) = 200 millimoles
Conversely, if you have 500 millimoles of a substance and want to know the equivalent moles, the conversion factor comes to the rescue:
500 millimoles * (1 mole / 1000 millimoles) = 0.5 moles
The conversion factor not only facilitates these unit conversions but also plays a crucial role in stoichiometric calculations, ensuring accurate determination of reactants and products involved in chemical reactions.
By understanding the concept of the conversion factor and its practical applications, you’ll become more proficient in handling mole-millimole conversions, unlocking a deeper comprehension of chemical quantities and reactions.
Molar Mass: Unraveling the Molecular Weight
In the realm of chemistry, understanding the molecular weight of substances is crucial, and this is where molar mass steps onto the stage. Just like a scale measures your weight, molar mass provides a measure for the weight of your molecules!
Defining Molar Mass
Molar mass is the mass of one mole of a substance. A mole, in turn, is a whopping number of particles, equivalent to approximately 6.022 × 10^23 particles. Imagine a humongous crowd of molecules, and that’s a mole!
Calculating Molar Mass
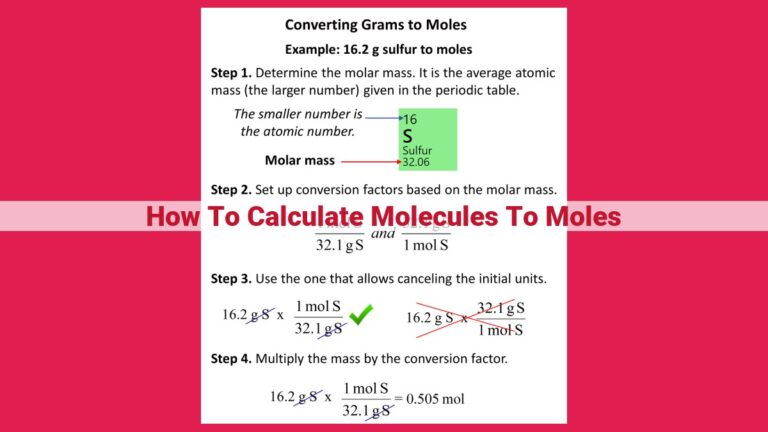
Calculating molar mass is quite straightforward. You simply add up the atomic masses of all the atoms in your molecule. The atomic masses are found on the periodic table. For instance, the molar mass of water (H2O) is 18.02 g/mol because it consists of two hydrogen atoms (1.01 g/mol each) and one oxygen atom (16.00 g/mol).
Molar Mass and Conversions
Molar mass plays a vital role in converting between mass and moles. Knowing the molar mass of a substance, you can easily determine how many moles are present in a certain mass or vice versa. For example, if you have 50 grams of water, you can calculate the number of moles by dividing the mass by the molar mass:
Number of moles = Mass / Molar mass
Number of moles = 50 g / 18.02 g/mol
Number of moles ≈ 2.77 moles
Significance of Molar Mass
Molar mass is not just a number; it’s a powerful tool in chemistry. By understanding molar mass, you can:
- Determine the molecular weight of substances
- Convert between mass and moles
- Calculate the number of particles in a sample
- Make accurate chemical calculations
Avogadro’s Number: Unlocking the Secrets of Quantifying Particles
In the realm of chemistry, where the world is made up of tiny atoms and molecules, scientists often face the daunting task of counting these microscopic entities. Enter Avogadro’s number, a remarkable constant that serves as a gateway to uncovering the hidden world of particles.
The Enigma of Counting Molecules
Imagine trying to count grains of sand on a vast beach or stars in the night sky. The sheer number would overwhelm our senses. Similarly, in chemistry, the number of atoms and molecules involved in reactions and substances is unfathomable.
Avogadro’s Brilliant Insight
In the early 19th century, Amedeo Avogadro, an Italian scientist, proposed a game-changing concept: that equal volumes of gases at the same temperature and pressure contain an equal number of molecules. This groundbreaking insight laid the foundation for understanding the nature of gases and the quantification of molecules.
The Constant of Six Trillion
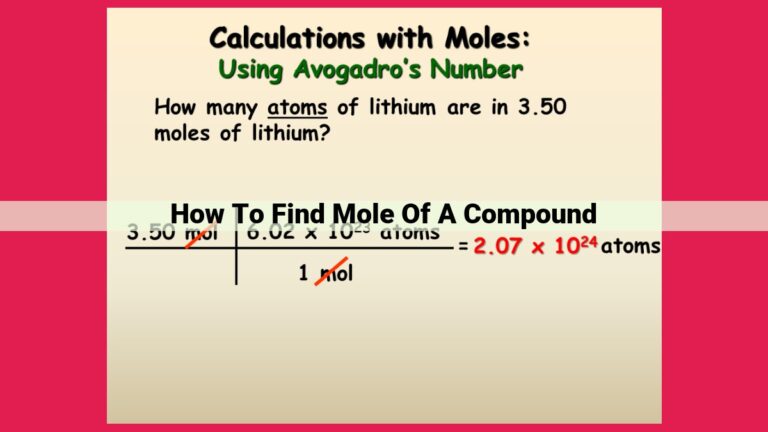
Through careful experimentation, Avogadro determined that 1 mole of any substance contains 6.022 × 10^23 particles. This staggering number, known as Avogadro’s number, is the key to unlocking the secrets of quantifying particles in the chemical realm.
Moles and Avogadro’s Number: A Powerful Duo
The mole, a fundamental unit in chemistry, measures the amount of substance present in a sample. When we combine the mole concept with Avogadro’s number, we gain the ability to convert between the mass of a substance and the number of particles it contains.
Counting Molecules like Magic
For instance, if we know the molar mass of a compound (the mass of 1 mole of that compound), we can use Avogadro’s number to determine the exact number of molecules or atoms in a given mass of that substance. This knowledge empowers scientists to understand the stoichiometry of reactions and the composition of various materials.
Avogadro’s number is a cornerstone of chemistry, providing scientists with an invaluable tool to quantify the countless particles that make up our world. By understanding the significance of this constant, we can unlock the secrets of chemical reactions, decipher the composition of substances, and delve deeper into the intricate workings of the universe at its microscopic level.