How To Convert Molecules To Moles: A Comprehensive Guide For Chemistry Experiments
To convert from molecules to moles, one must understand Avogadro’s number, which represents the number of particles in a mole (6.022 × 10^23). The mole relates to mass via molar mass, or the mass per mole of a substance. By dividing the mass of a substance by its molar mass, one can determine the number of moles. This conversion is essential for understanding quantitative relationships in chemistry, as the mole serves as a bridge between the microscopic and macroscopic scales, enabling the precise measurement and manipulation of matter.
Understanding Avogadro’s Number
- Define Avogadro’s number and its significance.
- Discuss the unit and magnitude of Avogadro’s number.
Understanding Avogadro’s Number: A Journey into the Microscopic World
Embark on a scientific adventure as we delve into the captivating world of chemistry and unravel the mysteries surrounding Avogadro’s number. This fundamental constant plays a pivotal role in understanding the microscopic realm, where matter exists in minuscule particles.
Defining Avogadro’s Number: The Building Block of Matter
Imagine a vast number, so large that it boggles the mind: 6.022 x 10^23. This is Avogadro’s number, the cornerstone of chemistry. It represents the exact quantity of atoms, molecules, or ions present in one mole of a substance, a fundamental unit used in chemical measurements.
The Magnitude and Unit: Understanding the Scale
Avogadro’s number is not merely a colossal number; it also carries a specific unit: moles per gram. This unit signifies the number of moles contained in every gram of substance. It provides a tangible way to quantify the immense number of particles present in even the smallest samples.
Setting the Stage: The Intertwined Concepts
To fully grasp Avogadro’s number, we must understand its interconnectedness with two other key chemistry concepts: molar mass and the mole. These concepts form a trinity, essential for navigating the microscopic world and deciphering the quantitative relationships that govern chemical interactions.
Molar Mass: The Mass Equivalent of a Mole
The molar mass of a substance represents the mass of one mole of that substance, expressed in grams per mole. It acts as a bridge between the microscopic world of atoms and molecules and the macroscopic world of grams and kilograms.
The Mole: Measuring Matter at a Microscopic Level
The mole is a unit that quantifies amount of substance. One mole of any substance contains exactly 6.022 x 10^23 particles of that substance. This concept allows us to measure and compare the quantities of different substances on a level playing field.
Unveiling the Interconnections: A Tapestry of Concepts
Avogadro’s number, molar mass, and the mole are inextricably linked. Molar mass provides the conversion factor between grams and moles, while Avogadro’s number establishes the exact number of particles present. Together, these concepts form the bedrock of quantitative chemistry, enabling us to understand the composition, properties, and behavior of matter at the atomic and molecular levels.
Molar Mass: The Mass Equivalent of a Mole
In the vast world of chemistry, we navigate the microscopic realm where matter exists in immeasurable quantities. To decipher this intricate world, scientists have devised a bridge between the macroscopic and the minuscule – Avogadro’s number, the cornerstone of our understanding of the mole and molar mass.
The mole is the unit of measurement for the amount of a substance. It is defined as the amount of substance that contains exactly Avogadro’s number of atoms, molecules, ions, or other elementary particles. Avogadro’s number, an astronomical figure of 6.022 * 10^23, represents the staggering number of particles in one mole of any substance. It serves as a universal conversion factor, allowing us to traverse the vast chasm between the macroscopic and microscopic realms.
Molar mass, on the other hand, is the mass of one mole of a substance. It is expressed in grams per mole (g/mol) and serves as a crucial parameter in quantitative chemical analysis. The molar mass of an element is determined by the sum of the atomic masses of its constituent atoms, as specified in the periodic table. This invaluable resource provides a wealth of information, including the molar mass of each element, making it an indispensable tool for chemists.
Molar mass plays a pivotal role in stoichiometry, the branch of chemistry that deals with the quantitative relationships between reactants and products in chemical reactions. By utilizing the molar mass of a substance, chemists can convert between the mass and the amount of that substance, facilitating precise calculations and ensuring accurate experimental outcomes.
In essence, molar mass is the mass equivalent of a mole, providing a bridge between the macroscopic and microscopic scales. It is a fundamental concept underpinning the understanding of chemical quantities, empowering chemists to quantify and manipulate matter with precision, unlocking the secrets of the molecular world.
The Mole: Measuring Matter at a Microscopic Level
In the realm of chemistry, understanding the microscopic world is essential. This is where the concept of the mole comes into play, a fundamental unit that allows us to quantify matter at the atomic and molecular level.
The mole is defined as the amount of substance that contains exactly 6.02214076 × 1023 entities, whether they be atoms, molecules, ions, or electrons. This astonishing number is known as Avogadro’s number, and it serves as a bridge between the microscopic and macroscopic scales.
The relationship between the mole, Avogadro’s number, and molar mass is crucial. Molar mass is the mass of one mole of a substance. It is measured in grams per mole (g/mol) and provides a convenient way to convert between the mass and the number of particles in a given sample.
For example, the molar mass of sodium chloride (NaCl) is 58.44 g/mol. This means that one mole of NaCl contains exactly 58.44 grams, and that 58.44 grams of NaCl contains 6.02214076 × 1023 molecules of NaCl.
The mole is an invaluable tool in chemistry. It allows us to:
- Quantify the amount of reactants and products in chemical reactions
- Determine the concentration of solutions
- Calculate the empirical and molecular formulas of compounds
- Understand the relationship between the properties of substances and their composition
By comprehending the mole and its interconnections with Avogadro’s number and molar mass, we gain a deeper understanding of the microscopic world and the quantitative relationships that govern chemical interactions.
Converting Molecules to Moles: A Practical Guide
In the realm of chemistry, understanding the interplay between molecules and moles is crucial. While molecules represent discrete units of matter, moles provide a quantifiable measure of a substance’s amount. To delve deeper into the quantitative relationships in chemistry, converting molecules to moles becomes essential.
Why Convert Molecules to Moles?
Converting molecules to moles is a necessary step when dealing with large quantities of matter. It allows scientists to work with manageable units that are easier to calculate and compare. For instance, instead of counting individual water molecules, chemists prefer to work with moles of water, which represent a known number (Avogadro’s number) of molecules.
Step-by-Step Conversion Instructions
-
Determine the Substance’s Molecular Weight: The first step is to identify the substance’s molecular weight. This value represents the sum of the atomic weights of all atoms in the molecule. For instance, the molecular weight of water (H2O) is 18 grams per mole.
-
Divide Mass by Molecular Weight: Measure the mass of the substance in grams. Divide this mass value by the substance’s molecular weight to obtain the number of moles.
Example Problem
Suppose you have 54 grams of sodium chloride (NaCl). To convert this mass to moles:
- Sodium chloride’s molecular weight is 58.44 grams per mole.
- Divide 54 grams by 58.44 grams per mole: 54 g / 58.44 g/mol = 0.923 moles of NaCl
This means that the 54 grams of sodium chloride contain 0.923 moles of NaCl molecules.
Converting molecules to moles is a fundamental operation in chemistry. By following the step-by-step instructions, scientists can efficiently determine the amount of a substance and facilitate calculations involving chemical reactions, stoichiometry, and other quantitative aspects of chemistry.
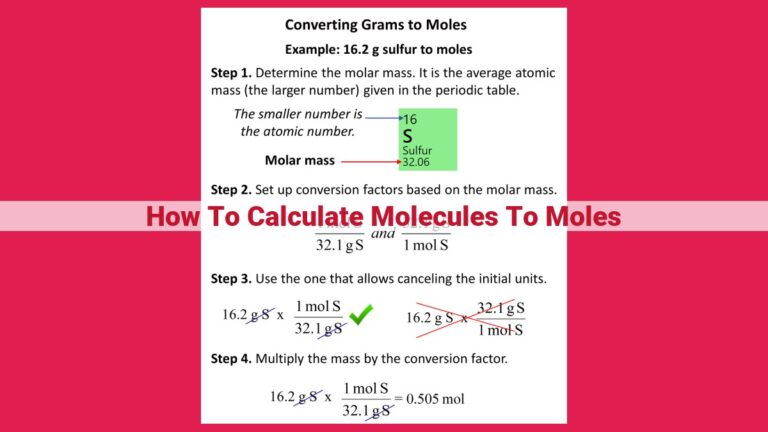
Example Problem: Calculating Moles from Mass
Let’s delve into a practical example to solidify our understanding of converting molecules to moles. Suppose you have 5.00 g of sodium chloride (NaCl) and want to determine the number of moles of NaCl present.
Step 1: Find the Molar Mass of NaCl
First, we need to know the molar mass of NaCl. This value tells us the mass of 1 mole of the substance. We can find the molar mass of NaCl by adding up the atomic masses of sodium (Na) and chlorine (Cl) from the periodic table:
Molar mass of NaCl = Atomic mass of Na + Atomic mass of Cl
= 22.99 g/mol + 35.45 g/mol
= 58.44 g/mol
Step 2: Convert Mass to Moles
Now we have the molar mass and the mass of NaCl. To convert mass to moles, we divide the mass by the molar mass:
Number of moles of NaCl = Mass of NaCl / Molar mass of NaCl
= 5.00 g / 58.44 g/mol
= **0.0855 moles of NaCl**
Therefore, 5.00 g of sodium chloride contains 0.0855 moles of NaCl. This conversion is critical for understanding the exact number of particles (atoms, molecules, or ions) present in a substance, allowing us to perform precise calculations in various chemical applications.
Interconnections of Avogadro’s Number, Molar Mass, and the Mole
The concepts of Avogadro’s number, molar mass, and the mole are fundamental to understanding the quantitative relationships in chemistry. These concepts are intricately interconnected and provide a framework for measuring and manipulating matter at the microscopic level.
Avogadro’s number, represented by the symbol Nₐ, is a constant that defines the number of particles (atoms, molecules, or ions) present in one mole of a substance. It is an enormous number, approximately *6.022 x 10²³, providing a bridge between the macroscopic and microscopic scales.
Molar mass is the mass of one mole of a substance expressed in grams. It represents the mass equivalent of Avogadro’s number of particles. Molar mass serves as a crucial conversion factor, allowing chemists to translate between the mass and number of particles in a sample.
The mole, symbolized as mol, is the SI unit of amount of substance and represents a specific quantity of particles equal to Avogadro’s number. The mole concept provides a convenient way to handle large numbers of particles, effectively simplifying chemical calculations.
These three concepts are interconnected in the following ways:
- One mole of any substance contains Nₐ particles, as defined by Avogadro’s number.
- The molar mass of a substance is the mass of one mole of that substance, expressed in grams.
- The mass of a sample can be converted to the number of moles using the molar mass as a conversion factor: Moles = Mass (g) / Molar Mass (g/mol)
This interplay between Avogadro’s number, molar mass, and the mole enables chemists to perform various quantitative calculations. For instance, they can determine the number of molecules in a sample, calculate the mass of a specific number of particles, or determine the concentration of a solution.
These concepts are essential tools in the study of chemistry, providing a solid foundation for understanding the behavior of matter and facilitating accurate and efficient chemical calculations.