Convert Molecules To Moles: A Comprehensive Guide
To convert molecules to moles, utilize Avogadro’s number (6.022 x 10^23 molecules/mol) as a conversion factor. The molar mass, representing the mass per mole of a substance, aids in converting between mass and moles. Additionally, molecular formulas provide the composition of compounds, while percent composition indicates the mass percentages of elements. By understanding these concepts, you can convert molecules to moles using the following steps: Divide the number of molecules by Avogadro’s number to obtain the number of moles.
The Importance of Converting Molecules to Moles: A Journey into the Molecular World
In the realm of chemistry, understanding the relationship between molecules and moles is paramount. _Molecules, the fundamental building blocks of matter, represent discrete chemical entities, while _moles, a unit of measurement, quantify the number of these entities in a given sample. Converting molecules to moles is crucial for unraveling the mysteries of the molecular world and is a fundamental skill in the field of chemistry.
Understanding Key Concepts: The Foundation for Conversion
To accurately convert molecules to moles, it’s essential to grasp the following concepts:
-
Avogadro’s Number (Nₐ): This constant represents the number of entities (molecules, atoms, or ions) in one mole of a substance, providing the conversion factor between molecules and moles.
-
_Molar Mass: _The mass of one mole of a substance, molar mass allows us to convert between the mass and the number of moles of a given substance.
-
_Molecular Formula: _A symbolic representation of the elements and their respective ratios in a compound, the molecular formula provides information about the composition and identity of the compound.
Avogadro’s Number: The Gateway to Counting Molecules
Imagine you’re at a bustling market, surrounded by an overwhelming number of vendor stalls. How do you keep track of the countless items, each with its unique quantity? The answer lies in using a unit of measurement, like a dozen for eggs or a pound for apples.
Similarly, in the realm of chemistry, we have Avogadro’s number – a fundamental constant that serves as the conversion factor between the microscopic world of molecules and the macroscopic world of quantities we can measure.
Avogadro’s number, represented by the symbol **Nₐ, is an astounding value: 6.022 × 10$^{23}$. This number signifies that one mole of any substance contains exactly this many constituent particles, be it atoms, molecules, ions, or electrons.**
The mole is a crucial unit of measurement in chemistry, analogous to the dozen or the pound. It provides a convenient way to express large quantities of substances without having to deal with cumbersome numbers of individual particles.
Avogadro’s number acts as the bridge between the microscopic and macroscopic scales in chemistry. By utilizing this conversion factor, we can seamlessly convert between the number of molecules and the number of moles, enabling us to accurately measure and analyze chemical substances.
Molar Mass: The Bridge Between Mass and Moles
In the realm of chemistry, understanding the relationship between mass and moles is crucial for comprehending the composition and reactions of substances. Molar mass serves as the bridge that connects these two realms, enabling us to convert between the number of molecules and the mass of a substance.
Molar Mass: Unveiling the Mass of a Mole
Every compound possesses a unique molar mass, which represents the mass of one mole of that compound. One mole is defined as an astronomical number, 6.022 x 10^23, of entities. These entities can be atoms, molecules, ions, or electrons, depending on the substance.
Imagine a bag containing exactly one mole of sugar molecules, C12H22O11. The molar mass of sugar is approximately 342 grams per mole. This means that our bag of sugar contains 342 grams of sugar molecules, a colossal number that would fill a room!
Molar Mass in Practice: Formula Mass and Percent Composition
Molar mass finds immense utility in determining the formula mass of compounds. Formula mass is simply the sum of the atomic masses of all the atoms present in a molecule. For instance, the formula mass of C12H22O11 is 342 grams, which aligns perfectly with its molar mass.
Molar mass also plays a pivotal role in establishing the percent composition of compounds. Percent composition reveals the mass percentage of each element within a compound. By comparing the molar mass of an element to the formula mass, we can calculate the mass percentage of that element.
Molar mass is a fundamental concept that unlocks the mysteries of chemistry. It links the microscopic world of molecules to the macroscopic realm of mass, facilitating our comprehension of substance composition and chemical reactions. By revealing the relationships between mass and moles, molar mass empowers us to decipher the complexities of the chemical world.
Molecular Formula: The Chemical Address of Compounds
In the world of chemistry, compounds are like unique entities, each with its own distinct identity. Just as an address provides a precise location for a house, the molecular formula serves as the chemical address for a compound, revealing its precise composition. It’s like a blueprint, unveiling the exact number and arrangement of atoms that make up the compound’s structure.
Defining the Molecular Formula
The molecular formula is a symbolic representation that succinctly describes the types and quantities of atoms present in a single molecule of a compound. Each element is represented by its chemical symbol, while subscripts indicate the number of atoms of each element in the molecule. For example, the molecular formula of water, H2O, tells us that a single water molecule consists of two hydrogen (H) atoms and one oxygen (O) atom.
Molecular Formulas and Percent Composition
The molecular formula not only identifies the elements present in a compound but also provides valuable insights into its composition. By knowing the molecular formula, we can determine the percent composition, which represents the mass percentage of each element in the compound. For instance, the percent composition of water can be calculated by dividing the mass of hydrogen (2 x atomic mass of hydrogen) and the mass of oxygen (1 x atomic mass of oxygen) by the total mass of the water molecule and multiplying by 100.
Example: Determining Percent Composition
Consider the molecular formula of carbon dioxide, CO2. To determine its percent composition, we need to know the atomic masses of carbon (12.01 g/mol) and oxygen (16.00 g/mol).
- Mass of carbon in CO2: 12.01 g/mol x 1 = 12.01 g
- Mass of oxygen in CO2: 16.00 g/mol x 2 = 32.00 g
- Total mass of CO2: 12.01 g + 32.00 g = 44.01 g
Percent composition of carbon in CO2:
= (12.01 g / 44.01 g) x 100%
= 27.28%
Percent composition of oxygen in CO2:
= (32.00 g / 44.01 g) x 100%
= 72.72%
Therefore, carbon dioxide is composed of approximately 27.28% carbon and 72.72% oxygen.
Percent Composition: Unveiling the Chemical Fabric
Picture yourself as a detective tasked with analyzing a mystery substance. How do you determine its components and proportions? Percent composition is your trusty tool, providing a numerical breakdown of the elements that make up a compound. It’s a crucial concept in chemistry, offering valuable insights into a compound’s composition.
Percent composition reveals the mass percentages of each element in a compound. For instance, water, with its molecular formula H2O, comprises 11.11% hydrogen and 88.89% oxygen. This means that for every 100 grams of water, you’ll find 11.11 grams of hydrogen and 88.89 grams of oxygen.
The relationship between molecular formulas and percent composition is a dance of ratios. The molecular formula tells you the number of atoms of each element in the compound, while percent composition quantifies their mass contributions. So, knowing a compound’s molecular formula allows you to calculate its percent composition, and vice versa.
Percent composition plays a pivotal role in understanding chemical structures and properties. It helps chemists identify compounds, compare their compositions, and predict their reactivity. This information is invaluable in various fields, from material science to pharmaceuticals, enabling scientists to tailor materials with specific properties or design drugs with targeted therapeutic effects.
Stoichiometry: The Rosetta Stone of Chemical Reactions
In the grand tapestry of chemistry, where matter dances and transforms, stoichiometry emerges as the deciphering language of chemical reactions. It’s the key to unlocking the quantitative relationships between reactants and products, unveiling the secrets hidden within those intricate equations that govern our world.
Picture a chemical reaction as a harmonious symphony, where molecules collide and rearrange, driven by the relentless laws of nature. Stoichiometry provides the musical score for this symphony, dictating which molecules will react, in what proportions, and what products will emerge from the chaos.
At the heart of stoichiometry lies the concept of mole ratios. Just as a conductor guides the interplay of instruments, mole ratios orchestrate the dance of atoms and molecules. These ratios, derived from the balanced chemical equation, serve as blueprints for predicting the exact quantities of reactants and products involved in a reaction.
Consider the classic reaction between hydrogen and oxygen:
2H2 + O2 -> 2H2O
The mole ratio tells us that for every two moles of hydrogen molecules consumed, one mole of oxygen molecule is required. And for every two moles of hydrogen molecules reacted, two moles of water molecules are produced.
Stoichiometry transforms chemistry from a qualitative pursuit to a quantitative science, allowing us to predict and control the outcome of chemical reactions with precision. This knowledge empowers us to engineer new materials, develop efficient manufacturing processes, and tackle complex problems in fields such as medicine, energy, and environmental science.
In the symphony of chemistry, stoichiometry stands as the conductor, orchestrating the complex interactions of molecules and revealing the hidden harmonies of our universe.
Converting Molecules to Moles: A Step-by-Step Guide
In the realm of chemistry, the ability to convert molecules to moles is a fundamental skill that unlocks a world of understanding and precision. By embarking on this journey of conversion, we gain the power to decipher the language of chemical reactions and unlock the secrets of molecular composition.
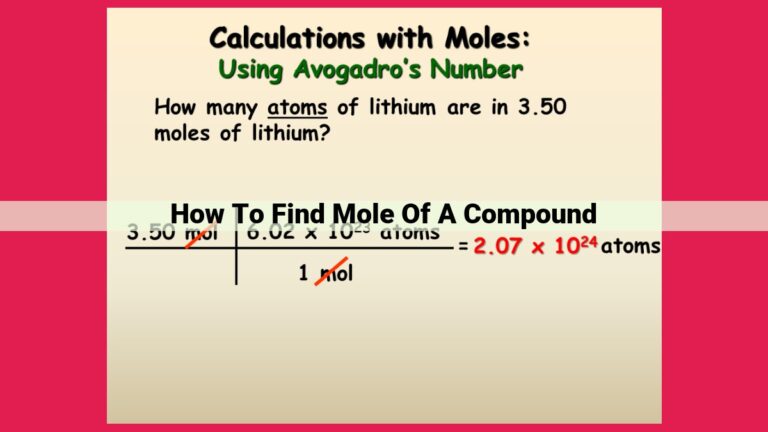
Step 1: Embracing Avogadro’s Number
At the heart of this conversion lies Avogadro’s number, a magical constant of 6.022 x 10^23 that serves as a bridge between the microscopic world of molecules and the macroscopic world of moles. A mole, a unit of measurement in chemistry, represents a gargantuan number of particles, approximately equal to the number of atoms in 12 grams of carbon-12.
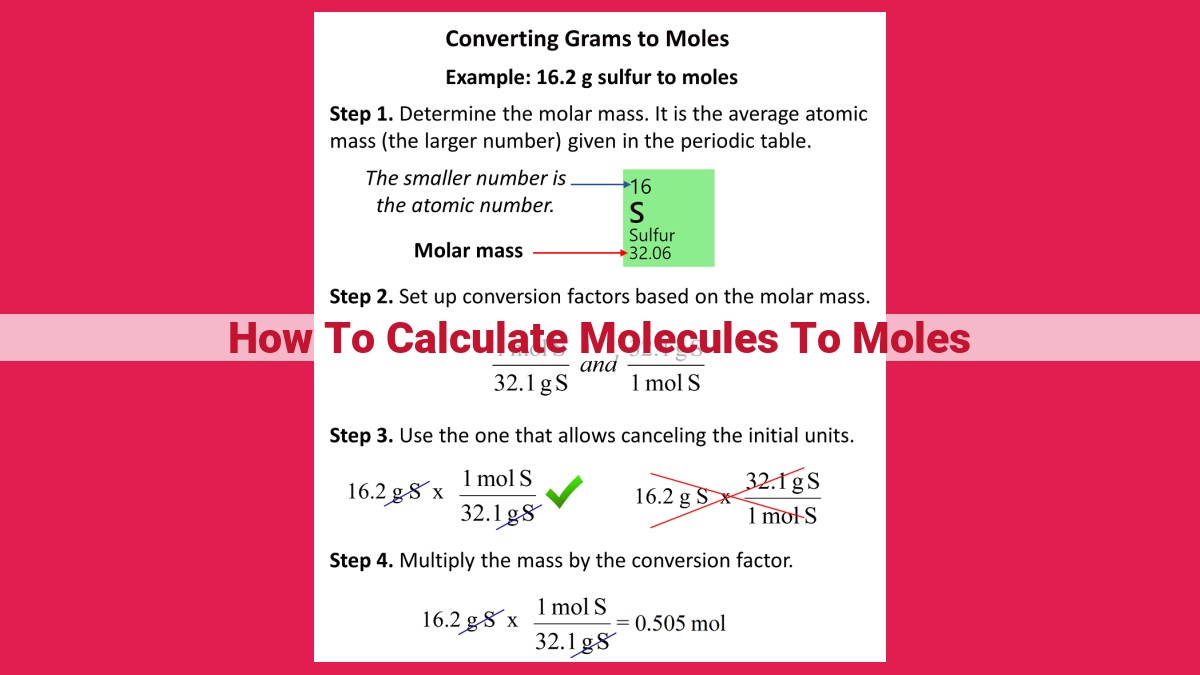
Step 2: Unveiling Molar Mass, the Mass-Mole Translator
Molar mass, a property unique to each substance, serves as the translator between mass and moles. It represents the mass of one mole of a substance expressed in grams. To determine the molar mass, simply add the atomic masses of all the atoms in the molecular formula. Armed with molar mass, we can now effortlessly convert between grams and moles.
Step 3: Master the Puzzle of Molecular Formulas
A molecular formula, like a secret code, reveals the arrangement and number of atoms in a compound. By deciphering this code, we can determine the percent composition, which unveils the mass percentages of each element in the molecule. This knowledge serves as a vital tool in understanding the composition and properties of compounds.
Step 4: Precision in Stoichiometry, the Language of Reactions
Stoichiometry, the language of chemical reactions, provides a roadmap for predicting the quantitative relationships between reactants and products. By using mole ratios, stoichiometry enables us to orchestrate balanced chemical equations and determine the exact amounts of reactants and products involved in reactions.
Step 5: Calculating Moles from Molecules
Now, let’s put our understanding to the test with a step-by-step guide to converting molecules to moles:
- Count the number of molecules, using experimental techniques or scientific notation.
- Divide the number of molecules by Avogadro’s number (6.022 x 10^23 molecules per mole).
- The result is the number of moles of the substance.
For instance, if we have 10^20 molecules of water (H2O), we can calculate the number of moles as follows:
Moles of H2O = (10^20 molecules) / (6.022 x 10^23 molecules/mole)
Moles of H2O = 1.66 x 10^-4 moles
Mastering the conversion between molecules and moles is a gateway to unraveling the intricacies of chemical reactions and molecular composition. With this knowledge, we become fluent in the language of chemistry, unlocking a world of possibilities in scientific research, technological advancements, and the understanding of the natural world.