Convert Atoms To Grams: A Comprehensive Guide For Unit Conversions

To convert atoms to grams, start with atomic mass to determine the mass of individual atoms. Combine atomic masses to calculate molecular mass for molecules. Convert molecular mass to molar mass, which represents the mass of one mole of a substance. Avogadro’s number provides a bridge between the number of entities and their mass. Use conversion factors, such as grams to moles, to navigate between different units. By following these steps, you can accurately convert from the microscopic scale of atoms to the macroscopic scale of grams.
Atomic Mass: The Bedrock of Mass Conversions
Understanding the Building Blocks of Matter
In the realm of chemistry, mass plays a pivotal role. And at the heart of this concept lies atomic mass, the cornerstone of understanding the average mass of an element. Each element’s atomic mass represents the weighted average of the masses of its naturally occurring isotopes, which are atoms of the same element with varying numbers of neutrons.
Isotopic Mass: A Tale of Neurons
Isotopes are like siblings, sharing the same atomic number but differing in their neutron count. These neutrons add to the mass of the atom without altering its chemical behavior. Thus, the isotopic mass of an element takes into account the contribution of each isotope and their relative abundances.
Calculating Atomic Mass: A Balancing Act
The atomic mass of an element is not merely the average of its isotopic masses. It’s a delicate balance, weighted by the relative abundances of each isotope. This intricate calculation ensures that the atomic mass accurately reflects the average mass of the element as it exists in nature.
Molecular Mass: Combining Atoms into Molecules
Determining the mass of a molecule, the fundamental building block of matter, is crucial for understanding its composition and behavior. This concept, known as molecular mass, quantifies the combined mass of all the atoms within a molecule.
To determine the molecular mass, we start with the molecular formula of the compound. This formula provides the number and type of atoms present in each molecule. For example, the molecular formula of glucose is C₆H₁₂O₆, indicating that each glucose molecule is composed of six carbon atoms, twelve hydrogen atoms, and six oxygen atoms.
Next, we calculate the atomic mass of each element in the formula. Atomic mass represents the average mass of an element’s naturally occurring isotopes, weighted by their relative abundance. For example, the atomic mass of carbon is approximately 12.011 amu, and the atomic mass of hydrogen is approximately 1.008 amu.
Finally, we multiply the atomic mass of each element by the number of atoms of that element present in the molecule. For example, in glucose, there are six carbon atoms, so we multiply the atomic mass of carbon (12.011 amu) by six. Similarly, for hydrogen atoms (12 x 1.008 amu) and oxygen atoms (6 x 16.000 amu).
By summing the products of the atomic masses and the number of atoms, we obtain the total molecular mass of the compound. In the case of glucose, the molecular mass is approximately 180.156 amu.
The molecular mass provides valuable insights into the composition and properties of the compound. It allows us to understand the mass ratio of different elements within the molecule and compare the masses of different molecules. Moreover, it is a crucial parameter in determining the molar mass of a compound, which further enables calculations involving the amount of substance present.
Molar Mass: Bridging the Gap between Mass and Amount
In the realm of chemistry, we often encounter quantities that span vast scales, from the tiny atoms that make up matter to the colossal amounts of substances used in industrial processes. To navigate this vastness, chemists rely on a fundamental concept known as molar mass.
Defining Molar Mass
Molar mass, simply put, is the mass of one mole of a substance. A mole, in turn, is defined as a specific number of elementary entities, be they atoms, molecules, ions, or electrons. This number, known as Avogadro’s number, is an astounding 6.022 × 1023.
Equivalence to Molecular Mass
For molecular compounds, molar mass is closely related to molecular mass. Molecular mass refers to the sum of the atomic masses of all atoms present in a molecule. However, molar mass takes this concept one step further by expressing the molecular mass in terms of grams.
Significance of Molar Mass
Molar mass plays a crucial role in determining the amount of substance present in a given mass. By knowing the molar mass of a compound, we can convert between mass and amount and vice versa. This knowledge is essential in various chemical calculations, such as:
- Determining the number of molecules present in a sample
- Calculating the concentration of solutions
- Balancing chemical equations
- Predicting the yield of chemical reactions
Molar mass serves as a bridge between the mass and amount of a substance, allowing us to navigate the vastness of the chemical world. It is a fundamental concept that empowers chemists to unravel complex chemical phenomena and make precise calculations.
Avogadro’s Number: Unveiling the Microscopic Realm
In the realm of chemistry, understanding the relationship between the mass of substances and their amount is crucial. One key concept in this endeavor is Avogadro’s number, which serves as a bridge between the macroscopic and microscopic worlds.
Defining Avogadro’s Number
Avogadro’s number, denoted by _N_A, is an astounding value: 6.022 x 1023. It represents the precise number of elementary entities (atoms, molecules, or ions) present in one mole of a substance. This number is named after the Italian scientist Amedeo Avogadro, who proposed the concept in the early 19th century.
The Mole as a Gateway
The mole, abbreviated as mol, is the SI unit for measuring amount of substance. One mole of a substance contains exactly N_A entities of that substance. By defining the mole in terms of Avogadro’s number, chemists have established an essential conversion factor between the number of entities and their corresponding mass.
Conversions with Avogadro’s Number
Avogadro’s number enables conversions between the number of entities and their mass. For example, if we know the mass of a substance in grams, we can use Avogadro’s number to determine the number of entities present. Conversely, if we know the number of entities, we can calculate the mass in grams. This conversion is essential in various chemical calculations, such as stoichiometry and limiting reagents.
Importance of Avogadro’s Number
Avogadro’s number is a fundamental constant in chemistry, facilitating mass-to-amount conversions and opening up the microscopic world. It provides a direct link between the observable properties of substances at the macroscopic level and their constituent particles at the microscopic level. Understanding Avogadro’s number is essential for comprehending and manipulating chemical reactions and processes.
Conversion Factors: Navigating the Units Maze
When delving into the realm of chemistry, we often encounter a myriad of units that describe the abundance and properties of substances. To decipher these units and perform accurate calculations, we rely on conversion factors, which are essential tools for navigating the “units maze.”
Dimensional Analysis and Conversion Factors
Dimensional analysis is a technique that enables us to manipulate units to ensure that our calculations make sense. Conversion factors are the key to this process. They are ratios of equivalent units, such as grams to moles or moles to molecules. By multiplying a value by the appropriate conversion factor, we can convert it from one unit to another.
Case Study: Mass Conversions
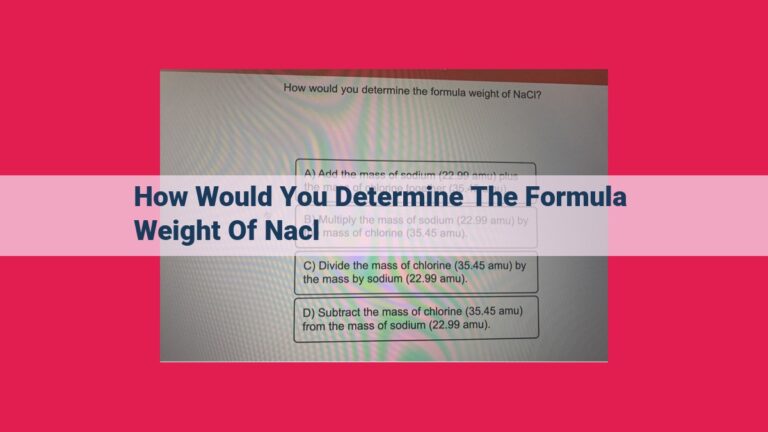
For instance, consider a problem where we need to convert 50 grams of sodium chloride (NaCl) to moles. We can use the conversion factor:
1 mole NaCl = 58.44 grams NaCl
To convert grams to moles, we multiply by the reciprocal of the conversion factor:
50 grams NaCl x (1 mole NaCl / 58.44 grams NaCl) = 0.855 moles NaCl
Similarly, if we have 0.25 moles of calcium carbonate (CaCO3) and need to determine its mass, we can use the conversion factor:
1 mole CaCO3 = 100.09 grams CaCO3
Multiplying by the conversion factor gives us:
0.25 moles CaCO3 x (100.09 grams CaCO3 / 1 mole CaCO3) = 25.02 grams CaCO3
Conversion factors are indispensable tools in chemistry, allowing us to bridge the gap between different units and ensure accurate calculations. By understanding and applying these factors, we can navigate the complexities of chemical measurements with ease.