Comprehensive Guide To Understanding Valence Electrons: Role In Chemistry And Importance For Br
Best Outline for Blog Post
-
Introduction to Valence Electrons
- Explain what valence electrons are and their role in chemical bonding.
- Describe the importance of understanding valence electrons.
-
Concept of Valence Electrons
- Define valence electrons and their location in an atom.
- Explain how the period number in the periodic table relates to valence electrons.
- Discuss the role of valence electrons in chemical reactivity.
-
Related Concept: Orbitals
- Explain what orbitals are and how they relate to valence electrons.
- Describe how the configuration of outermost orbitals determines the number of valence electrons.
-
Related Concept: Atomic Number
- Explain the concept of atomic number and its relevance to valence electrons.
- Describe the formula for calculating valence electrons using atomic number.
-
Focus: Number of Valence Electrons in Br
- State the atomic number of bromine (Br).
- Identify the inner-shell electron configuration of bromine.
- Calculate the number of valence electrons in bromine using the formula provided.
-
Explanation of Valence Electrons in Br
- Describe the distribution of valence electrons in bromine’s outermost orbital.
- Explain the arrangement of electrons according to the Pauli exclusion principle.
-
Conclusion
- Summarize the key points about valence electrons, orbitals, and atomic number.
- Emphasize the significance of understanding these concepts for chemical bonding.
- Explain what valence electrons are and their role in chemical bonding.
- Describe the importance of understanding valence electrons.
Understanding the Significance of Valence Electrons
In the captivating world of chemistry, understanding the intricacies of atoms is crucial. Among these intricacies, valence electrons take center stage, playing a pivotal role in the symphony of chemical interactions.
Valence electrons, the outermost electrons of an atom, wield the power to forge chemical bonds, the invisible connections that hold atoms together. Like tiny dancers whirling around the nucleus, these electrons dance to the tune of their energy levels, influencing the atom’s _chemical reactivity—its ability to participate in reactions with other atoms.
Delving deeper, the realm of orbitals emerges as the ethereal space where valence electrons reside. These orbitals, with their unique shapes and energy levels, dictate the arrangement and behavior of _valence electrons. The outermost orbitals, with their higher energies, house the _valence electrons, determining their availability for bonding.
The periodic table, a map of the chemical elements, offers a guiding light in deciphering the number of valence electrons. The period number, the row an element occupies, holds the key. Each period represents a layer of orbitals, with the outermost layer dictating the _valence electrons.
Every atom carries a distinctive atomic number, its passport to the chemical world. This number, the number of protons in the nucleus, also serves as a roadmap to valence electrons. The formula Atomic number — innermost shell electron configuration unveils the coveted number of _valence electrons.
In the context of bromine (Br), an element with an atomic number of 35, its innermost electron configuration is [Ar]18. As the outermost orbital accommodates a maximum of 8 valence electrons, _bromine emerges with 7 valence electrons. These valence electrons, arranged in a specific configuration according to the _Pauli exclusion principle, dance and interact, shaping bromine’s chemical behavior and bonding capabilities.
Unveiling the secrets of valence electrons, orbitals, and _atomic number empowers us to comprehend the symphony of chemical reactions and forge a deeper understanding of the molecular world around us.
Concept of Valence Electrons: Unlocking the Secrets of Chemical Bonding
Imagine yourself as a detective, embarking on a thrilling adventure to uncover the mysteries of the atomic world. Valence electrons, our elusive target, hold the key to understanding the intricate dance of chemical elements.
Valence electrons reside in the outermost shell of an atom, like the boundary line between the atom’s core and the outside world. Their position determines the atom’s ability to interact with its neighbors. Think of them as the social butterflies of the atomic realm, eager to form bonds and create new substances.
The period number in the periodic table offers a valuable clue in predicting the number of valence electrons. Each period represents a new energy level, and the group number (also known as the family) indicates the number of electrons in the outermost shell. For instance, elements in Group 1 have one valence electron, while those in Group 18 (noble gases) have a full complement of eight valence electrons, making them chemically inert.
Understanding valence electrons is crucial for deciphering the language of chemical reactivity. Atoms with unpaired valence electrons, like lonely hearts searching for a match, are more likely to react with other atoms to fill their outer shells and achieve stability. The number and arrangement of valence electrons determine the types of bonds an element can form, whether it be ionic, covalent, or metallic.
Valence Electrons: The Building Blocks of Chemical Bonding
In the world of chemistry, understanding the behavior of atoms is crucial for unraveling the mysteries of chemical bonding. One key aspect of atomic behavior lies in valence electrons, the electrons that reside in the outermost shell of an atom and determine its chemical properties.
What are Valence Electrons?
Imagine an atom as a miniature solar system, with the nucleus, the central core, representing the sun and the electrons orbiting it like planets. Valence electrons are those electrons that occupy the outermost orbits, the farthest from the nucleus. These electrons are the most active and play a pivotal role in chemical bonding.
The number of valence electrons is a crucial factor in determining an atom’s chemical reactivity. Atoms with more valence electrons tend to be more reactive because these electrons are more likely to interact with electrons from other atoms.
The Role of Orbitals
Orbitals are the specific regions around an atom where electrons are most likely to be found. Each orbital can hold a maximum of two electrons. The configuration of the outermost orbitals determines the number of valence electrons.
For instance, an atom with a single electron in its outermost orbital has one valence electron. An atom with two electrons in its outermost orbital has two valence electrons, and so on.
The Case of Bromine (Br)
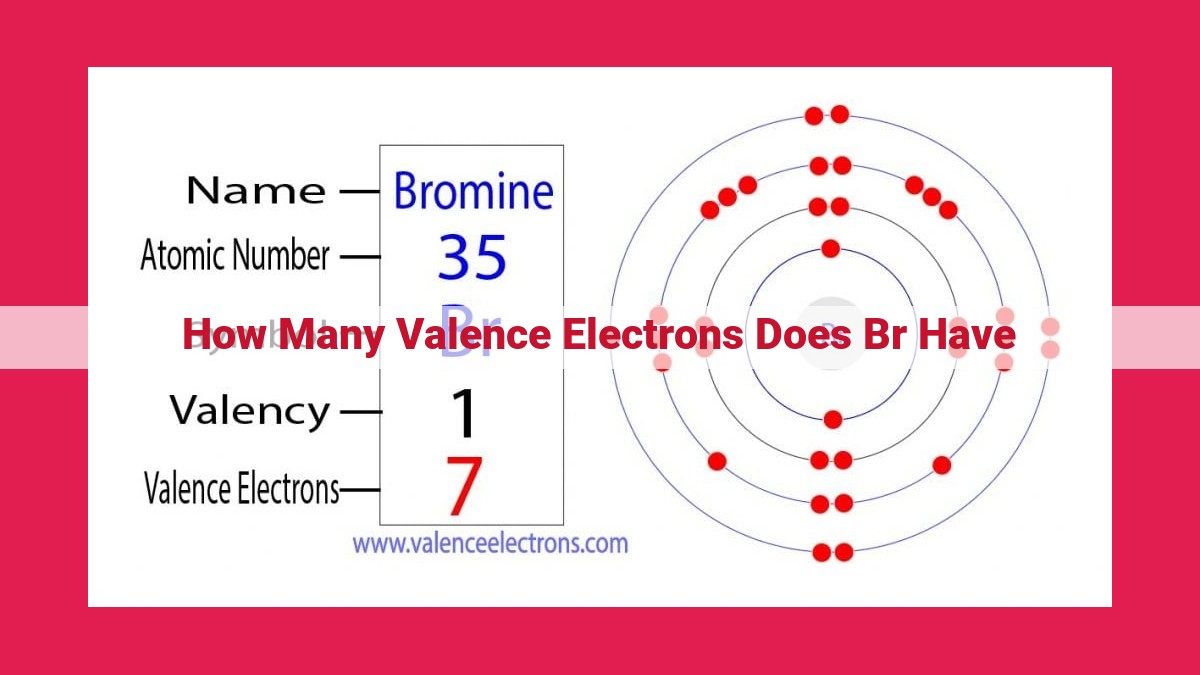
To illustrate the concept of valence electrons, let’s consider the element bromine (Br). Its atomic number is 35, indicating that it has 35 electrons. The inner-shell electron configuration of bromine is [Ar] 3d¹⁰ 4s² 4p⁵. This means that bromine has five valence electrons, as the 4p orbital can accommodate up to six electrons.
The distribution of valence electrons in bromine’s outermost orbital is 4p⁵. This means that there are five electrons occupying the p orbitals, with one electron in each of the three subshells (px, _py, and _pz).
Understanding the number and arrangement of valence electrons is essential for understanding chemical bonding. By manipulating the interactions between these outer electrons, we can predict and control the formation of new molecules and compounds.
Unlocking the Secrets of Valence Electrons: A Journey into the Realm of Chemical Bonding
In the bustling world of chemistry, the concept of valence electrons holds a profound significance. These elusive electrons, residing in an atom’s outermost energy level, play a crucial role in determining its chemical reactivity and bonding behavior. Join us on an exciting expedition to unravel the mysteries of valence electrons and their captivating influence on the world of chemistry.
The Concept of Valence Electrons
Imagine an atom as a miniature solar system, with the nucleus acting as the sun and electrons orbiting like planets. Valence electrons are those electrons that occupy the outermost orbit of an atom. They are like the messengers of the atom, responsible for interacting with other atoms and forming chemical bonds.
The number of valence electrons is directly related to an atom’s position in the periodic table. Elements in the same group or vertical column have the same number of valence electrons. This understanding empowers us to predict the chemical behavior of elements based on their location in the table.
Role of Valence Electrons in Chemical Reactivity
Valence electrons have an insatiable desire to achieve a stable configuration of eight electrons. When atoms interact, they exchange or share valence electrons to fulfill this desire. This electron exchange or sharing forms the basis of chemical bonding, the force that holds atoms together to form molecules and compounds.
Atomic Number and Valence Electrons
Each element is uniquely identified by its atomic number, which reveals the number of protons in its nucleus. Surprisingly, the atomic number also holds the key to determining the number of valence electrons an atom possesses.
The secret lies in the formula for valence electrons:
Number of Valence Electrons = Atomic Number – Number of Core Electrons
Core electrons are the electrons occupying the inner orbits of an atom. By subtracting the number of core electrons from the atomic number, we can unveil the number of valence electrons.
Valence electrons, the orchestrators of chemical bonding, are the key to unlocking the secrets of chemical reactivity. Understanding their concept, relationship with the periodic table, and atomic number empowers us to comprehend the fundamental principles of chemistry and decipher the fascinating world of molecules and compounds.
Remember, the mastery of valence electrons is not merely a collection of facts but a testament to the profound interconnectedness of the natural world. May this journey spark your curiosity and inspire you to explore the wonders of chemistry that lie ahead.
Valence Electrons: Unraveling the Chemical Identity of Bromine
Begin your journey by understanding valence electrons, the outermost electrons in an atom that play a pivotal role in chemical bonding. They determine an element’s reactivity and serve as the key to deciphering its chemical behavior.
Bromine: A Chemical Puzzle
Let’s focus on bromine (Br), an element known for its reddish-brown hue and its presence in various compounds. To grasp its chemical nature, we need to determine the number of valence electrons it possesses.
Unveiling Bromine’s Electron Configuration
Every atom has a specific number of electrons, and bromine is no exception. Its atomic number, represented as Z, is 35. This number reveals the total number of electrons in a neutral bromine atom.
The electron configuration of bromine is crucial in understanding its valence electrons. The electrons are arranged in shells or energy levels, with each shell accommodating a set number of electrons. The outermost shell, also known as the valence shell, holds the valence electrons.
For bromine, the electron configuration can be written as 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁵. This configuration indicates that there are 7 valence electrons in the outermost shell.
Calculating Valence Electrons: A Shortcut
Determining the number of valence electrons can be simplified using a formula:
Valence electrons = Atomic number – (Number of electrons in inner shells)
For bromine, with an atomic number of 35 and 28 electrons in the inner shells, the calculation is:
35 – 28 = 7 valence electrons
The Significance of Valence Electrons in Bromine
Understanding the number of valence electrons in bromine is vital for predicting its chemical behavior. With 7 valence electrons, bromine tends to form 1 covalent bond or share 7 electrons to attain a stable electron configuration.
This knowledge is essential for comprehending the formation of various bromine compounds, including those used in flame retardants, dyes, and medications.
Explanation of Valence Electrons in Br
Understanding Bromine’s Valence Electrons
Bromine, an element with atomic number 35, holds a significant place in the periodic table due to its unique arrangement of electrons. Its valence electrons, responsible for chemical interactions, play a pivotal role in determining its reactivity and properties.
Bromine possesses a total of 7 valence electrons distributed in its outermost energy level. These electrons occupy three p-orbitals, denoted as px, py, and pz. The Pauli Exclusion Principle, a fundamental law of quantum mechanics, dictates that no two electrons within an atom can have the same set of four quantum numbers, including spin. This principle governs the organization of electrons in bromine’s valence shell.
In the px orbital, bromine houses two electrons with opposite spins, as indicated by the notation:
- 1s2 2s2 2px2
The py orbital also accommodates two electrons with contrasting spins:
- 1s2 2s2 2py2
The remaining three valence electrons reside in the pz orbital, arranged in a unique pattern:
- 1s2 2s2 2pz3
This configuration underscores the principle that no two electrons within the same orbital can have the same spin orientation. The three electrons in the pz orbital exhibit parallel spins, represented by three arrows pointing either all up or all down.
Significance of Valence Electrons
Bromine’s valence electrons are crucial for understanding its chemical properties. Their involvement in bonding determines the element’s affinity for forming covalent bonds with other atoms. By sharing valence electrons, bromine can achieve a stable electron configuration, thus forming stable molecules and compounds.
In conclusion, the arrangement and behavior of valence electrons in bromine provide valuable insights into its chemical attributes. Understanding these concepts enhances our comprehension of atomic structure and lays the foundation for exploring the intricacies of chemical bonding.