Periodic Table: A Comprehensive Guide To Element Organization
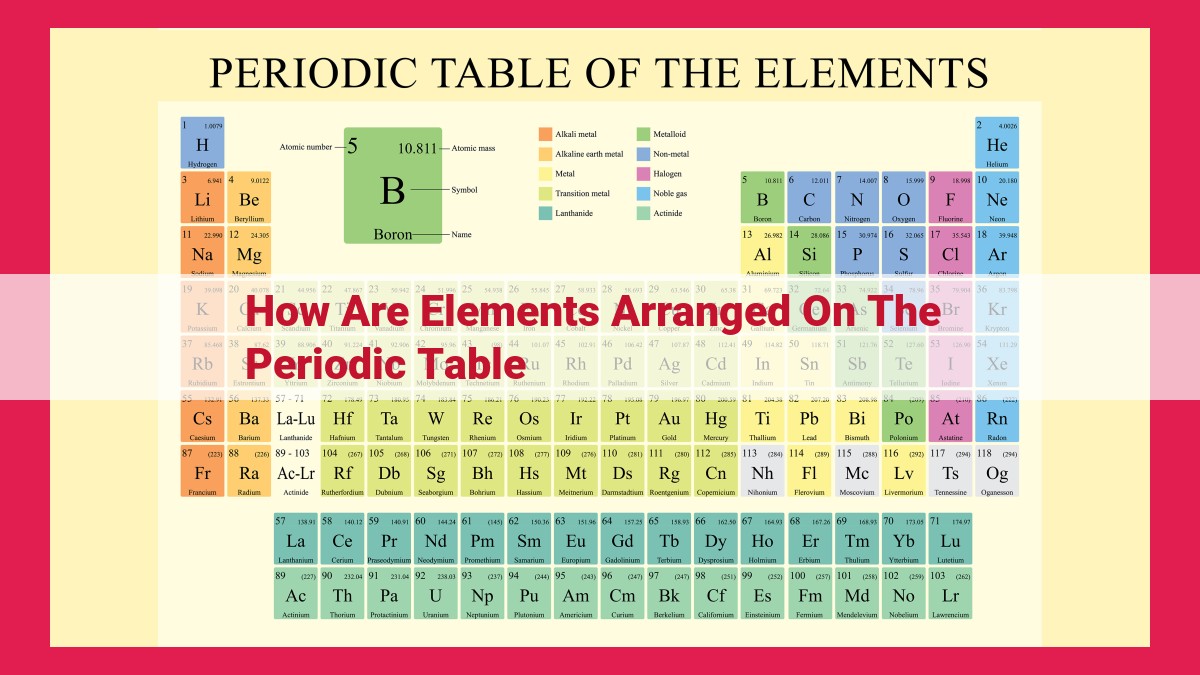
The periodic table organizes elements in a systematic tabular format based on their atomic number, which determines their position, and properties. Elements are arranged into horizontal rows (periods) representing energy levels and vertical columns (groups) containing elements with similar chemical and physical properties. The arrangement reflects their valence electrons, which play a crucial role in determining chemical bonding and reactivity.
Define the periodic table as a tabular arrangement of elements based on their atomic number and properties.
The Periodic Table: Unraveling the Enigma of Elements
Imagine a vast array, a tabular landscape meticulously organized, where each element occupies its rightful place. This is the periodic table, a testament to the intricate order that governs the universe of matter.
At the heart of this table lies a fundamental concept: atomic number. Each element is distinguished by the unique number of protons residing in the very center of its atoms. This number serves as the guiding light, determining an element’s position on the table.
As we delve further, we encounter the notion of atomic mass, a measure that embraces the diversity of isotopes within each element. Isotopes, subtle variations of the same element, possess identical atomic numbers but differ in their neutron count. This intricate interplay gives rise to the concept of atomic weight, a weighted average that captures the essence of an element’s isotopic composition.
The Periodic Table: A Guide to Elemental Organization
In the fascinating tapestry of science, the periodic table stands as a beacon of order, unveiling the intricate relationship between chemical elements. Envision a celestial map charting the universe of elements, with each atomic number acting as a celestial coordinate.
Atomic number, the defining characteristic of an element, embodies the very essence of its identity. It represents the number of protons residing within the enigmatic nucleus of an atom’s core. Protons, positively charged particles, determine an element’s position on the periodic table, like stars illuminating their celestial abode.
Similar to celestial bodies orbiting in harmony, electrons, with their tireless dance, govern the chemical destiny of elements. Electrons, negatively charged entities, reside in energy levels, which encircle the nucleus like planetary rings. The outermost energy level, adorned with a captivating ensemble of electrons, holds the key to an element’s valence.
Delving into the Fascinating World of Atomic Mass: An Average Tale of Isotopes
In the realm of chemistry, the periodic table stands as a vibrant tapestry of elements, each with its unique characteristics. Among these properties, atomic mass takes center stage as a measure of an element’s average mass, revealing the intricate story of its isotopic composition.
Isotopes, nature’s diverse twins, are atoms of the same element that share an identical atomic number, but differ in their neutron count. This difference in neutron count results in subtle variations in the element’s mass, leading to the concept of isotopic abundance.
Within an element’s isotopic family, each isotope contributes to the average mass, weighted by its relative abundance. The atomic mass, therefore, provides a composite snapshot of the mass of an element, considering all its isotopic variants.
Take, for instance, carbon, the building block of life. Carbon has three naturally occurring isotopes: carbon-12, carbon-13, and carbon-14. Carbon-12, the most abundant, accounts for about 98.9% of the total carbon atoms. Carbon-13, its heavier cousin, constitutes 1.1%. And carbon-14, a radioactive isotope used in carbon dating, is a mere trace component.
The atomic mass of carbon, 12.011, reflects this isotopic composition. It is not a whole number because it is an average, weighted by the abundance of each isotope.
Understanding atomic mass opens a window into the variegated world of isotopes, unraveling the intricate tales of their natural abundance and the impact they have on an element’s average mass.
Explain isotopic composition and atomic weight as related concepts.
Understanding Atomic Number and Mass
In the realm of chemistry, the periodic table stands as an invaluable guide, organizing the elements of the universe in a way that unveils their secrets and relationships. Among the fundamental concepts that underpin the periodic table is the understanding of atomic number and mass.
Atomic Mass: Unveiling the Weight of Matter
Every element on the periodic table possesses a distinctive atomic mass, a numerical value that reflects the average weight of its atomic forms. These atomic forms, known as isotopes, are variations of the same element that differ slightly in their neutron count. Neutrons, along with protons, reside in the heart of an atom, its nucleus.
Isotopic Composition: Exploring the Atom’s Inner Diversity
The atomic mass of an element is determined by the abundance of its different isotopes. Each isotope has its own unique isotopic composition, a measure of the relative proportions of these isotopic variations. By understanding the isotopic composition of an element, scientists can gain insights into its nuclear properties and its behavior in chemical reactions.
Atomic Weight: Unifying Isotopes into a Single Value
The atomic weight of an element is a weighted average, taking into account the isotopic composition and the atomic masses of its isotopes. This single value represents the average mass of all the naturally occurring isotopes of that element. Atomic weight is a crucial parameter in various calculations, such as determining the molar mass of compounds and predicting the mass-to-charge ratio of ions in mass spectrometry.
Define periods as horizontal rows representing layers of energy levels.
Understanding Periodic Table: A Guide to Elemental Organization
The Periodic Table: A Symphony of Elements
In the realm of chemistry, there exists a fascinating tapestry of elements, each with its unique identity and characteristics. The periodic table is the master organizer of these elements, arranging them in a grid-like structure that unravels the secrets of their behavior.
Horizontal Harmony: Periods
Imagine the periodic table as a grand piano with horizontal rows, called periods. Each period represents a layer of energy levels within the atoms of the elements. As we move across a period from left to right, the number of electrons in the outermost energy level increases. These outermost electrons, known as valence electrons, play a crucial role in shaping the chemical properties of the elements.
Vertical Unity: Groups
Now, let’s turn our attention to the vertical columns, known as groups. Elements within a group share a common number of valence electrons, resulting in remarkable similarities in their chemical behavior. Group 1 elements, for instance, are highly reactive metals, while Group 18 gases are exceptionally stable and unreactive.
Energy Levels and Electron Configuration
Delving deeper into the periodic table, we discover the concept of electron configuration, the arrangement of electrons in an atom’s energy levels. The electron configuration of an element dictates its reactivity. Noble gases, for instance, have a complete electron configuration, making them chemically inert. In contrast, alkali metals and halogen elements have incomplete electron configurations, leading to their high reactivity.
Unveiling the Secrets of the Periodic Table: A Guide to Elemental Organization
Every element that exists has a unique place on the periodic table, a tabular arrangement that organizes these building blocks of matter based on their atomic number.
The atomic number is like an element’s fingerprint, revealing the number of protons in its nucleus. The higher the atomic number, the more protons an element has. These protons determine an element’s position on the periodic table, running from hydrogen with one proton to oganesson with 118.
Beneath the atomic number, you’ll notice another important property: atomic mass. This reflects the average weight of an element’s isotopes, variations of the same element with differing numbers of neutrons. Every element is made up of isotopes, which all share the same number of protons but have different numbers of neutrons, resulting in slightly varying masses.
Vertical Columns: Groups of Similar Beings
Now, let’s delve into the vertical columns of the periodic table known as groups. Groups consist of elements sharing similar chemical and physical characteristics. The magic behind this harmony lies in their valence electrons, those crucial electrons residing in the outermost energy level of an atom.
Valence electrons play a starring role in the chemical world, as they’re primarily responsible for the bonds that hold atoms together. Elements in the same group share the same number of valence electrons, giving them similar chemical behaviors.
For instance, the alkali metals (Group 1) are renowned for their high reactivity due to their single valence electron. They eagerly react with other elements to achieve a stable electron configuration.
Noble gases (Group 18), on the other hand, are the epitome of chemical stability. Their valence electron shells are filled to the brim, making them reluctant to participate in chemical reactions.
This group-based understanding of elements’ behaviors empowers us to make informed predictions about their chemical interactions and the properties of the compounds they form. The periodic table truly is a roadmap guiding us through the vast and intriguing world of elements.
Explain valence electrons as the electrons in an atom’s outermost energy level.
Valence Electrons: The Key Players in Chemical Behavior
Before delving into the fascinating world of valence electrons, it’s essential to set the stage by understanding the structure of an atom. Picture an atom as a tiny universe, with a dense core called the nucleus and orbiting clouds of electrons resembling satellites.
Within these electron clouds, the valence electrons play a crucial role. These are the electrons that occupy the outermost energy level of an atom, the level that’s furthest away from the nucleus. It’s these valence electrons that predominantly participate in chemical bonding, the process by which atoms interact and form molecules.
Imagine the valence electrons as the social butterflies of the atomic world, eager to connect with their neighbors. The number of valence electrons an atom possesses determines its chemical behavior and influences its bonding preferences. Atoms with similar numbers of valence electrons tend to have similar chemical properties, forming the basis for the organization of elements in the periodic table.
Understanding valence electrons is paramount in comprehending the diverse chemical reactions that shape our world. They dictate the reactivity of elements, their ability to form bonds, and the properties of the compounds they create. Without these vital electrons, the world of chemistry would be a much duller place indeed.
Valence Electrons: The Key to Chemical Bonding and Reactivity
Imagine yourself at a bustling party filled with people of all personalities and interests. The room is buzzing with conversations and laughter, but there’s a subtle undercurrent of energy that connects everyone: the desire to interact. In the world of chemistry, this desire to interact is driven by the valence electrons, the electrons in an atom’s outermost energy level.
Just like partygoers seeking companionship, valence electrons are eager to form bonds with other atoms to achieve a stable configuration. These bonds form the foundation of chemical bonding, the molecular glue that holds together everything around us, from water to proteins.
The number of valence electrons an atom has determines its chemical reactivity. For instance, atoms with a full complement of valence electrons, like noble gases, are content to keep to themselves, exhibiting low reactivity. On the other hand, atoms with incomplete valence shells, such as alkali metals and halogens, are highly reactive, eagerly seeking bonding partners to fill their outermost energy levels.
Explain the concept of electron configuration as the arrangement of electrons in energy levels.
Electron Configuration: Unraveling the Atomic Signature
In the fascinating realm of chemistry, the periodic table reigns supreme, a masterpiece of organization that unveils the secrets of the elements. Within this tabular arrangement lies a treasure trove of information about their atomic number, mass, and behavior. Yet, amidst this symphony of numbers, one concept stands out as a pivotal determinant of an element’s chemical dance: electron configuration.
Electron configuration, in essence, is the symphony of electrons within an atom, a meticulously orchestrated dance that governs an element’s reactivity and bonding properties. These electrons, like celestial bodies, reside in specific energy levels around the atom’s nucleus, forming concentric shells.
The arrangement of these electrons is not random but follows a set of rules dictated by quantum mechanics. The first shell, closest to the nucleus, can accommodate a maximum of two electrons, the second shell eight, and so forth. As each shell fills, a new one emerges, creating a hierarchy of energy levels.
Each electron within these shells possesses a unique energy level, with those in the outermost shell having the highest energy. These outermost electrons, known as valence electrons, are the key players in determining an element’s chemical behavior. They are the diplomats that interact with other atoms, forming bonds and creating the vibrant tapestry of chemical interactions.
Understanding electron configuration is akin to decoding a secret language, unlocking the insights into an element’s reactivity. It explains why noble gases, with their full outermost shells, are inert and reluctant to form bonds. Conversely, alkali metals, with a single valence electron, are highly reactive, eager to shed their lone electron and achieve stability.
Electron configuration also sheds light on the properties of halogens, renowned for their ability to gain electrons and complete their outermost shell. They are the chemical matchmakers, forming strong bonds with other elements to achieve electronic harmony.
In the vast expanse of chemistry, electron configuration serves as an essential guide, revealing the inner workings of atoms and unraveling the secrets of their interactions. By deciphering this atomic signature, we gain a profound understanding of the chemical world and its myriad phenomena.
The Symphony of Electrons: Unlocking the Secrets of Reactivity
In the vast cosmic dance of elements, electrons play a mesmerizing melody, dictating the reactivity and behavior of each atomic player. The periodic table, a tabular masterpiece, arranges these elements based on their atomic number, the number of protons residing in their nuclei. This strategic placement unveils a path to understanding the mesmerizing relationship between electron configuration and reactivity.
Electrons, the invisible forces that orbit the atomic nucleus, are distributed across energy levels, with the outermost energy level, known as the valence level, holding the key to reactivity. Valence electrons are the eager participants in the dance of chemical bonding, determining whether an element will embrace or spurn a partnership.
Let’s explore this relationship through the lens of diverse element groups:
Noble Gases: The Inert Spectators
Like celestial bodies content in their cosmic solitude, noble gases possess a full complement of valence electrons. This contentment renders them unreactive, the epitome of chemical indifference. Their stable electron configuration acts as an invisible shield, protecting them from the temptations of bonding.
Alkali Metals: The Eager Electron Givers
Alkali metals, on the other hand, are the eager beavers of the periodic table, readily willing to donate their solitary valence electron. Their ceaseless desire to achieve a stable octet of valence electrons drives their relentless pursuit of chemical bonding.
Alkaline Earth Metals: The Balanced Participants
Alkaline earth metals follow in the footsteps of alkali metals, but with a slight twist. These gracious elements possess two valence electrons, sharing them with partners to achieve a harmonious balance. Their affinity for bonding, though not as intense as that of alkali metals, is still apparent in their chemical endeavors.
Halogens: The Electron-Hungry Predators
Halogens stand on the opposite side of the electron spectrum, perpetually seeking to complete their valence electron octet. Their relentless pursuit transforms them into eager electron acceptors, forming stable compounds with other elements hungry for a partnership.
Transition Metals: The Versatile Chameleons
Transition metals embody the spirit of adaptability. Their electron configurations grant them the flexibility to adopt multiple oxidation states, giving them a remarkable range of reactivity. From forming colored complexes to catalyzing essential reactions, transition metals are the versatile chameleons of the periodic table.
Understanding the intricate interplay between electron configuration and reactivity empowers us to predict the behaviors of elements, paving the way for harnessing their potential in diverse fields from medicine to industry. The periodic table serves as a symphony of electrons, orchestrating the dance of reactivity that shapes our world.