Comprehensive Guide To Mixtures: Identifying, Classifying, And Separating
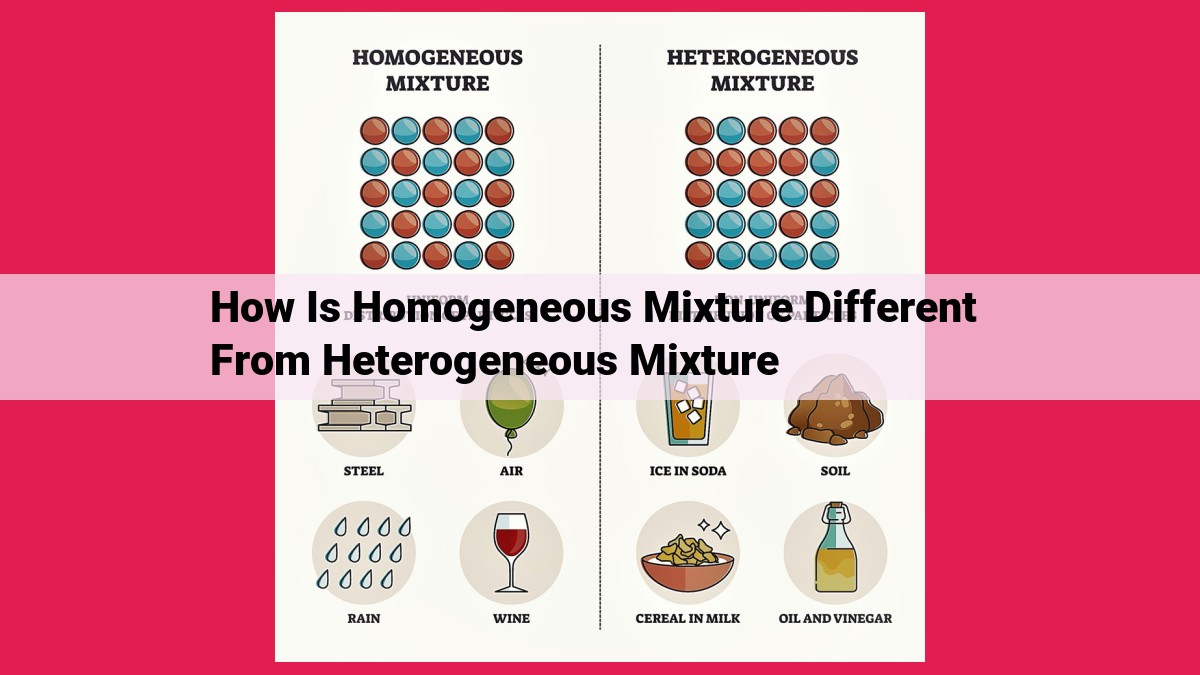
Mixtures comprise elements/compounds retaining their identities. Homogeneous mixtures appear uniform throughout, with evenly distributed components. Heterogeneous mixtures exhibit visible variations due to unevenly distributed components. The Tyndall effect, which scatters light in heterogeneous mixtures, aids in their distinction. Particle size plays a crucial role in classifying mixtures, with smaller particles contributing to homogeneity. Separation methods vary based on mixture type, with homogeneous mixtures requiring more advanced techniques due to their uniform composition.
Understanding Mixtures: A Tale of Uniting Ingredients
In the realm of chemistry, mixtures play a pivotal role, combining elements or compounds while preserving their distinct identities. Think of a delicious salad, where each ingredient retains its flavor and texture, creating a harmonious blend. Just as in a salad, mixtures exist in various forms, each with its unique characteristics.
Homogeneous Mixtures: The Uniform Blend
In the world of homogeneous mixtures, every part looks and behaves like the whole. Imagine a glass of perfectly mixed lemonade; no matter which sip you take, you’ll encounter the same delightful balance of sweet and sour. These mixtures appear uniform throughout, as their components are evenly distributed and intermingled.
Heterogeneous Mixtures: A Patchwork of Parts
On the other side of the spectrum, heterogeneous mixtures present a more diverse appearance. Think of a bowl of trail mix, where nuts, chocolate chips, and dried fruit cohabit in a medley of colors and textures. These mixtures are not uniform; you can clearly observe their constituent parts.
Diving into the World of Mixtures
Types of Mixtures
Mixtures, fascinating combinations of elements or compounds, grace our lives in countless forms. But not all mixtures are created equal. They come in two distinct flavors: homogeneous and heterogeneous.
Homogeneous Mixtures: A Seamless Blend
Imagine a perfectly stirred cup of coffee, its coffee grounds and water harmoniously united. This is a homogeneous mixture. Its components are so evenly distributed that you can’t discern any individual particles. From every angle, you’ll encounter the same consistent appearance.
Heterogeneous Mixtures: A Patchwork of Parts
In contrast, heterogeneous mixtures are like a colorful mosaic. Sand mixed with pebbles, for instance, presents a visible patchwork of different components. The particles remain distinct, creating a heterogeneous blend.
Distinguishing the Two
The key to distinguishing homogeneous from heterogeneous mixtures lies in their appearance. Homogeneous mixtures exhibit uniformity, while heterogeneous mixtures boast diversity. It’s like the difference between a blank canvas and a masterpiece painted with a thousand brushstrokes.
Appearance: A Tale of Size and Distribution
The appearance of mixtures hinges on the size and distribution of their constituent particles. Smaller particles create a more homogeneous blend, while larger particles give rise to heterogeneity.
Take a suspension, like muddy water, for example. Its particles are large enough to scatter light, creating the cloudy appearance. On the other hand, a solution, such as salt dissolved in water, has minuscule particles that distribute evenly, resulting in a transparent solution.
Tyndall Effect: Unmasking Mixtures
The Tyndall effect provides a handy tool to differentiate between homogeneous and heterogeneous mixtures. When a beam of light passes through a mixture, its path can be illuminated by the scattering of light by the particles. Heterogeneous mixtures, with their larger particles, produce a more intense scattering, casting a visible beam. Homogeneous mixtures, on the other hand, scatter light less effectively, leaving the beam relatively invisible.
Appearance of Mixtures: How Particle Size and Distribution Affect Their Look
Mixtures, a fascinating combination of elements or compounds, come in various forms, each exhibiting unique appearances. The size and distribution of particles play a crucial role in shaping these appearances. Let’s delve into how these factors transform the visual characteristics of mixtures.
Particle Size: From Clear to Cloudy
Picture a clear glass filled with water. When sugar is added, the water remains transparent because sugar molecules are so small that they cannot be detected by the naked eye. The mixture appears homogeneous, meaning its components are evenly distributed.
Contrast this with a glass of water filled with sand. Sand particles are much larger and remain suspended in the water, making the mixture turbid. The appearance is heterogeneous, indicating a non-uniform distribution of components.
Particle Distribution: Evenly Spread or Clumped Together
Imagine two mixtures, both containing the same amount of salt and water. In the first mixture, salt particles are evenly dispersed throughout the water, resulting in a uniform appearance. This is known as a homogeneous mixture.
In the second mixture, salt particles **clump together*, creating visible aggregates. The mixture appears heterogeneou with distinct regions of high and low salt concentration.
Examples of Appearance Variations
- Milk: Milk is an emulsion, a mixture of fat droplets dispersed in water. These droplets are tiny enough to create a smooth, opaque appearance.
- Fog: Fog is a suspension of water droplets in air. The droplets are small enough to scatter light, giving fog its characteristic hazy appearance.
- Paint: Paint is a mixture of pigments suspended in a liquid medium. Larger pigment particles create a rough, textured appearance, while smaller particles result in a smooth, glossy finish.
The appearance of mixtures is a window into their composition and properties. By understanding how particle size and distribution influence appearance, we gain insights into the behavior and applications of these mixtures in various fields, from science and engineering to everyday products like food and cosmetics.
**The Tyndall Effect: A Distinctive Light-Scattering Phenomenon**
Immerse yourself in the fascinating world of mixtures and discover the profound insights offered by the Tyndall effect. This remarkable phenomenon illuminates the subtle differences between homogeneous and heterogeneous mixtures, providing a deeper understanding of their nature and properties.
The essence of the Tyndall effect lies in the way light interacts with particles suspended in a mixture. When a beam of light passes through a homogeneous mixture, the particles are uniformly distributed throughout the medium, causing minimal scattering. As a result, the light travels unimpeded, creating a clear and transparent solution. Think of a glass of water illuminated by sunlight; the light appears to pass through without any visible obstruction.
In contrast, a heterogeneous mixture presents a different scenario. The particles in such mixtures are larger and often unevenly distributed, resulting in significant scattering of light. As the light encounters these particles, it bounces and scatters in all directions, creating a hazy or opaque appearance. Picture a dusty room illuminated by a flashlight; the light bounces off the dust particles, casting a diffuse glow throughout the space.
The Tyndall effect serves as a powerful tool for distinguishing between homogeneous and heterogeneous mixtures. By observing the scattering of light, scientists and researchers can quickly determine the nature of a given mixture. Heterogeneous mixtures exhibit a visible Tyndall effect due to the presence of larger, dispersed particles that scatter light, while homogeneous mixtures remain clear and transparent because their particles are too small to cause significant scattering.
This phenomenon extends beyond the confines of the laboratory and finds practical applications in various fields. For instance, the Tyndall effect helps determine the presence of colloidal particles in a solution, which is crucial in fields such as medicine and nanotechnology. It aids in detecting the quality of milk, with fresh milk appearing more transparent than spoiled milk due to the Tyndall effect.
In summary, the Tyndall effect provides a fascinating insight into the world of mixtures. By analyzing the scattering of light, it allows us to differentiate between homogeneous and heterogeneous mixtures, unlocking a deeper understanding of their properties and applications.
Particle Size: The Key to Classifying Mixtures
In the realm of chemistry, mixtures play a crucial role in forming various substances around us. They are combinations of elements or compounds that exist without losing their unique properties. However, understanding the behavior of mixtures requires delving into their composition and characteristics, one of which is particle size.
Particle size, the physical dimensions of particles within a mixture, exerts a significant influence on their behavior and classification. When particles are of comparable size and evenly distributed throughout the mixture, homogeneity prevails (homogeneous mixture). In such mixtures, the composition remains consistent regardless of the sample size. On the contrary, heterogeneous mixtures exhibit uneven particle distribution and variations in composition across the sample.
The Tyndall Effect serves as a valuable tool in distinguishing homogeneous from heterogeneous mixtures. When a beam of light passes through a mixture, heterogeneous mixtures scatter the light due to particle size differences, creating a visible cloudy appearance. In contrast, homogeneous mixtures allow light to pass through without scattering, resulting in a transparent appearance.
Particle size also influences the separation methods used for mixtures. Filtration, a technique used to separate solids from liquids, relies on the size of particles. Larger particles, like sand, can be retained by a filter while smaller particles, such as ions, may pass through. Centrifugation, another versatile technique, employs centrifugal force to separate particles. By spinning the mixture at high speeds, heavier particles are pushed outward, allowing lighter particles to remain in suspension.
In conclusion, particle size plays a vital role in classifying and understanding mixtures. Homogeneous mixtures exhibit uniform particle distribution and consistent composition, while heterogeneous mixtures display uneven particle distribution and varied composition. The Tyndall Effect helps differentiate between the two types. Furthermore, particle size influences the choice of separation methods, as filtration and centrifugation depend on particle size to effectively separate components. Recognizing the significance of particle size thus provides a deeper understanding of the behavior and properties of mixtures in our everyday lives.
**Separation Methods for Homogeneous and Heterogeneous Mixtures**
When it comes to mixtures, understanding their properties is crucial for effective separation. Homogeneous mixtures, with their uniform composition, require different techniques compared to heterogeneous mixtures, where components are visibly distinct. Let’s delve into the diverse separation methods used for each type.
Homogeneous Mixtures
Due to their uniform nature, homogeneous mixtures demand more refined separation techniques. Distillation is a go-to method for separating liquids with different boiling points. By heating the mixture and condensing the vapor, the components can be collected separately. Chromatography, a highly versatile technique, separates mixtures based on their affinity for different surfaces or phases. This method finds applications in various fields, including chemistry and biochemistry.
Heterogeneous Mixtures
Unlike homogeneous mixtures, heterogeneous mixtures offer more straightforward separation options. Filtration is a simple yet effective technique that uses a filter to separate solids from liquids. The filter allows the liquid to pass through while trapping the solid particles. Decantation, another widely used method, involves carefully pouring off the liquid from a settled mixture, leaving the solid particles at the bottom. Magnetic separation, as the name suggests, utilizes magnets to separate magnetic materials from non-magnetic ones. This method is particularly useful for mixtures containing metal impurities.
Particle Size and Separation
The size of the particles in a mixture plays a significant role in determining the appropriate separation method. Larger particles can often be separated using physical methods like filtration or sieving, while smaller particles may require more specialized techniques like centrifugation or electrophoresis. Choosing the correct separation method based on particle size ensures efficient recovery of the desired components.
In conclusion, the separation of mixtures depends on their composition and particle size. Homogeneous mixtures require specialized techniques like distillation and chromatography, while heterogeneous mixtures can be separated using simpler methods like filtration and decantation. Understanding the principles behind these separation methods empowers you to effectively separate mixtures, unlocking the potential of these diverse combinations for various applications.