Comprehensive Guide To Drawing Oxygen In Detail For Depth And Realism
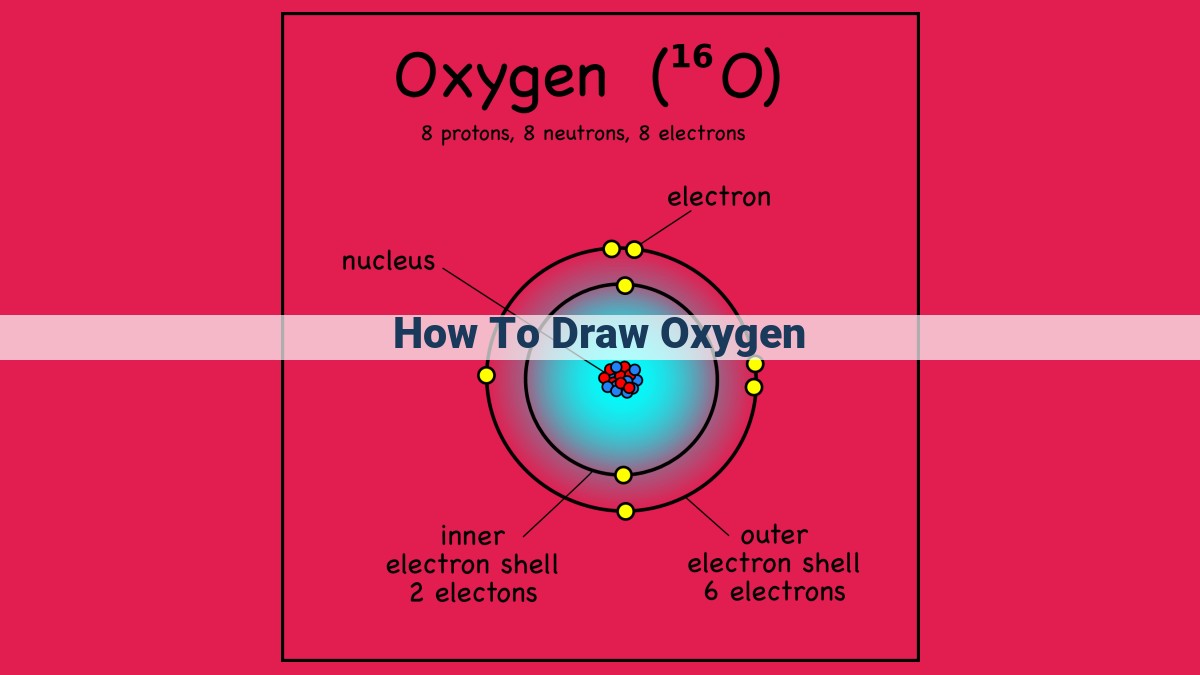
To draw oxygen, start with a side-on perspective for depth. Use circles for atoms and overlap them for volume. Mark the 8 valence electrons with electron dot diagrams. Connect atoms with lines for covalent bonds, labeling the 1.21 Å bond length. Indicate lone pairs with dots. Add highlights and shadows for realism, and vary lone pair size and placement for depth. Finally, add texture to atoms for enhanced details.
Introduction: Understanding Oxygen
- Define oxygen and explain its importance in biological processes.
Oxygen, the lifeblood of our world, plays a pivotal role in the intricate tapestry of biological processes. Without this vital element, the flame of life would flicker and extinguish. From the depths of our lungs to the photosynthetic marvels of plants, oxygen tirelessly fuels the myriad biochemical reactions that sustain and nourish our existence.
Understanding Oxygen’s Significance
Oxygen, the second most abundant element in the Earth’s atmosphere, is essential for cellular respiration, the process by which cells convert energy from nutrients into usable fuel. When we inhale, oxygen enters our lungs and embarks on a remarkable journey through our bloodstream to reach every nook and cranny of our bodies.
Inside cells, oxygen acts as a potent oxidant, reacting with various molecules to release energy. This process generates the adenosine triphosphate (ATP) that powers countless cellular activities, from muscle contractions to brain function. Without a steady supply of oxygen, cells would rapidly perish, and life as we know it would cease to be.
Perspective and Depth
- Discuss the importance of drawing oxygen from a side-on angle for depth.
- Explain the use of vanishing points to guide molecular lines.
Perspective and Depth
In the realm of scientific illustration, capturing the three-dimensional nature of molecules is crucial for conveying their structure and function. When it comes to oxygen, a vital molecule for life, drawing it from a side-on angle is of utmost importance for achieving depth.
Imagine yourself looking at an oxygen molecule straight on. You would see it as a flat, two-dimensional object, devoid of any visual cues to its three-dimensional form. However, by drawing the molecule from a side-on perspective, you introduce a sense of depth that allows viewers to appreciate its spherical shape.
In addition to the side-on angle, vanishing points play a significant role in guiding molecular lines and creating a cohesive three-dimensional representation. Vanishing points are imaginary points at infinity where parallel lines converge. By using vanishing points as references, you can ensure that the lines connecting the atoms in your oxygen molecule recede into the distance, creating a sense of perspective.
By mastering these techniques, you can elevate your scientific illustrations of oxygen molecules, transforming them from flat, two-dimensional representations into realistic, three-dimensional objects that accurately convey their structure and complexity.
Basic Shape and Volume: Capturing the Essence of Oxygen’s Form
In our quest to unravel the molecular world, we encounter the vital element oxygen. Its presence sustains life, enabling countless biological processes. To truly capture its essence, we must delve into the realm of artistic representation, where shape and volume take center stage.
Oxygen atoms, the building blocks of this essential element, are represented visually as perfect circles. These circles, when drawn with precision, convey the spherical nature of the atoms. However, to create a sense of depth and realism, we must go beyond simple circles.
By overlapping the circles, we create a subtle illusion of depth. This technique mimics the way oxygen atoms interact in space, their electron clouds intertwining to form a cohesive molecule. The overlapping areas create a sense of volume, adding a three-dimensional quality to our representation.
In this way, through the interplay of circles and overlapping forms, we can bring the invisible world of oxygen to life, giving it tangible shape and volume on the canvas of our art.
Electrons and Distribution: Unlocking the Secrets of Oxygen’s Valence
Every oxygen atom in our world holds a treasure chest of valence electrons – eight of them, to be exact. These electrons are the key to understanding oxygen’s unique chemical behavior.
To visualize these electrons, chemists use electron dot diagrams. Imagine a circle representing the oxygen atom’s nucleus, surrounded by eight dots – these are the valence electrons. They dance around the nucleus, like tiny planets orbiting a star.
Each electron is eager to find its place in the universe, sharing its orbit with other electrons. This sharing of orbits leads to the formation of chemical bonds – the building blocks of molecules.
Covalent Bonds: The Molecular Glue Holding Oxygen Atoms Together
Imagine a microscopic world where atoms, the fundamental building blocks of matter, dance around each other. Among these tiny dancers, oxygen atoms stand out, craving connection to form molecules. These connections, known as covalent bonds, are the invisible glue that holds them together, creating the vital oxygen molecules we breathe.
Picture two oxygen atoms, each with a treasure chest of eight valence electrons, the electrons eagerly seeking partners to share. As they approach each other, they hold out their valence electrons like tiny hands, ready to join forces. When these hands meet, they form a sigma bond, a covalent bond represented by a line connecting the atomic nuclei—the hearts of the atoms.
The distance between these atomic nuclei is crucial, affecting the molecule’s bond length. For oxygen-oxygen sigma bonds, this length is precisely 1.21 Ångströms (Å), a tiny measure equivalent to one ten-billionth of a meter. Accurately depicting this bond length is essential for scientific accuracy in molecular drawings.
By understanding and depicting these covalent bonds, we gain insights into the fundamental forces that shape the molecular world, from the oxygen we breathe to the countless molecules that make up our universe.
**Lone Pairs and Valence Electrons: The Guiding Light of Molecular Structure**
Within the realm of oxygen molecules, there exists a dance of electrons, each carrying an inherent charge that shapes their behavior. As you embark on your artistic journey, understanding these lone pairs and their valence electrons becomes paramount. These invisible forces govern the very essence of the molecule, guiding you towards a captivating depiction of molecular structure.
Oxygen atoms, like celestial dancers, possess a total of eight valence electrons — the electrons responsible for their interactions with the world. As they intertwine, these electrons form covalent bonds, akin to invisible threads that connect the atoms. However, not all electrons find their soulmate in bonding. Those that remain unattached, like solitary travelers, are known as lone pairs.
Representing lone pairs in your sketch is as simple as dotting them around the oxygen atoms, like little satellites orbiting a celestial body. These lone pairs are not to be underestimated, for they play a crucial role in determining the molecule’s overall structure and properties. Their presence can introduce an asymmetry to the molecule, breaking the symmetry that might otherwise exist.
Shading Techniques for Depth and Realism
In the realm of chemistry sketching, capturing the essence of molecules requires meticulous attention to detail. Shading plays a pivotal role in enhancing depth and infusing sketches with a sense of realism.
By strategically applying highlights and shadows, one can create the illusion of three-dimensionality, making molecules leap off the page. Highlights, the brightest areas, represent the regions where light strikes directly. They illuminate the edges of atoms, giving them a rounded appearance. Conversely, shadows, the darkest areas, depict the regions hidden from light. They recede into the molecule, creating a sense of depth.
To achieve smooth transitions between light and dark areas, gradients come into play. These subtle shifts in brightness mimic the gradual transitions of light in the real world. By blending highlights and shadows with gradients, you can eliminate harsh lines and amplify the molecule’s natural curves.
Mastering these shading techniques transforms mere sketches into dynamic representations of molecular structures. They add dimensionality to atoms, emphasize key features, and simulate the interplay of light and matter. Your drawings will transcend the limitations of flatness, captivating both the eyes and the mind.
Enhanced Details for Depth
When rendering oxygen molecules, capturing the intricate nuances adds visual impact and depth. Introduce texture to your atoms, giving them a realistic surface. This technique mimics the irregularities found in real molecules, creating a more authentic representation.
Varying the size and placement of lone pairs further enhances the illusion of three-dimensionality. Position lone pairs asymmetrically around each oxygen atom, avoiding a perfectly symmetrical arrangement. This asymmetry mimics the dynamic nature of lone pair electrons, orbiting the atom in different planes.
By incorporating these subtle enhancements, your oxygen molecule will transcend a mere drawing and become a visually compelling portrayal of a fundamental building block of life.