Compound F: Comprehensive Understanding For Industries, Research, And Applications
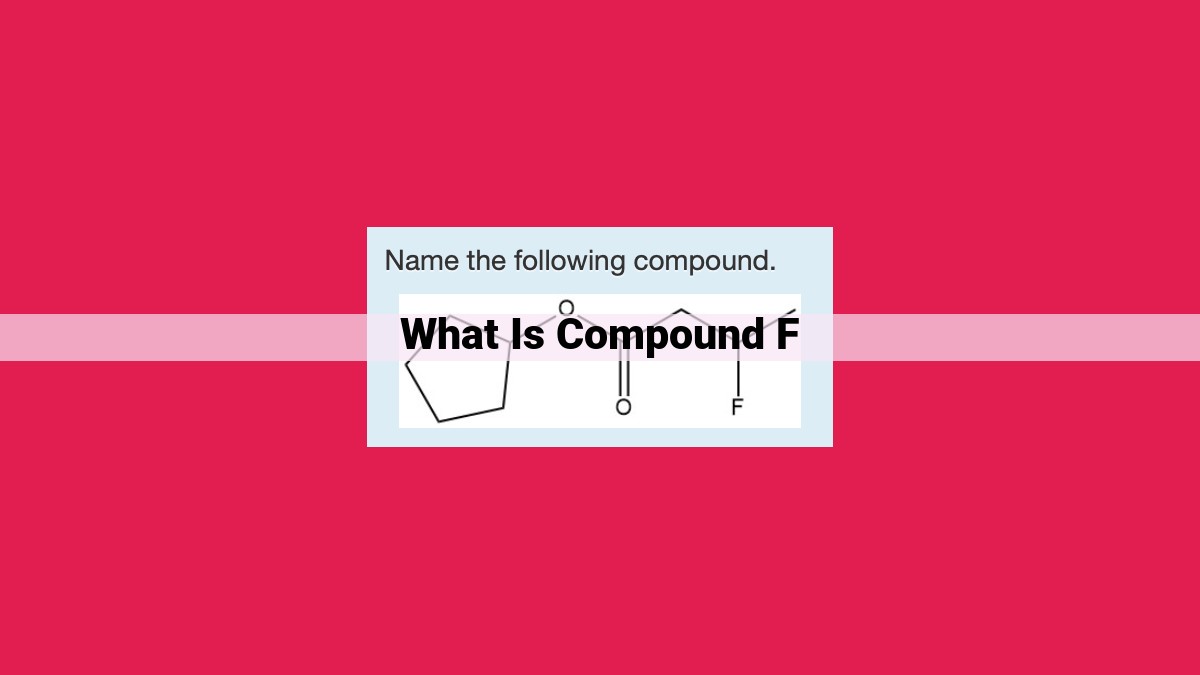
Compound F, a significant organic compound, plays crucial roles in various industries. Its molecular structure involves a distinct geometry, bonding, and isomerism. The stoichiometry and empirical formula define its elemental composition. Physical and chemical properties, including reactivity, provide insights into its behavior. Synthesis methods, involving organic chemistry techniques, enable its production. Compound F finds applications in food science, pharmaceuticals, and cosmetics. Understanding its toxicity, metabolism, biological activity, and environmental impact ensures safe and effective utilization.
Compound F: A Comprehensive Overview
In the realm of chemistry, a myriad of compounds play crucial roles in shaping our world. Among them is Compound F, a substance of immense significance that finds applications in a wide array of industries, from pharmaceuticals to cosmetics. In this article, we embark on a comprehensive exploration of Compound F, unraveling its chemical structure, properties, synthesis, and diverse uses.
Chemical Structure: Unraveling the Building Blocks
Compound F is an intricate molecule, its intricate structure a symphony of atoms bound together by chemical forces. We delve into the molecular geometry, bonding patterns, and fascinating world of isomers to decipher the secrets of this compound’s architecture.
Molecular Formula: Unraveling Elemental Composition
Beyond its structure lies the elemental composition of Compound F. We examine the stoichiometry, empirical formula, and molecular weight, gaining insights into the proportions and identities of the elements that constitute this compound.
Properties: Exploring Physical and Chemical Characteristics
The properties of Compound F, both physical and chemical, shed light on its behavior in various environments. We investigate its melting point, boiling point, solubility, reactivity, and other distinctive traits that define its interactions with the world around it.
Chemical Structure: Decoding the Building Blocks of Compound F
Step into the realm of molecular mysteries as we unravel the chemical structure of Compound F. It’s a journey that begins with molecular geometry, the spatial arrangement of its atoms like intricate puzzle pieces. Imagine a three-dimensional scaffold, defining the shape and character of this remarkable compound.
Next, we delve into the intricacies of chemical bonding, the forces that bind atoms together. Electrons, the tiny messengers of the chemical world, dance gracefully to form shared and lone pairs, creating a symphony of connections that hold the structure intact.
Finally, we encounter the concept of isomerism, a fascinating phenomenon where compounds with the same molecular formula exhibit different structures. Structural isomers differ in the arrangement of atoms, while stereoisomers share the same arrangement but differ in their spatial orientation. These subtle variations can have profound implications for the properties and behavior of Compound F.
As we uncover the building blocks of this intriguing substance, we gain deeper insights into its molecular architecture and the hidden forces that shape its destiny in the world of chemistry.
Molecular Formula: Unraveling Elemental Composition
Unveiling the Secrets of Compound F
In the world of chemistry, understanding the building blocks of a compound is paramount. The molecular formula of Compound F reveals its elemental composition, imparting crucial insights into its properties and behavior.
Stoichiometry: Counting the Atoms
Stoichiometry is the language of chemical proportions. It tells us the exact number of atoms of each element present in a compound. For Compound F, the stoichiometry of its molecular formula provides a precise roadmap to its atomic constituents.
Empirical Formula: The Simplest Ratio
The empirical formula represents the simplest whole-number ratio of the elements in a compound. It doesn’t specify the number of atoms, but rather the relative proportions. Compound F’s empirical formula offers a glimpse into its basic elemental composition.
Molecular Weight: Massing the Molecules
Finally, the molecular weight of Compound F is the sum of the atomic weights of its constituent atoms. This value helps us determine the mass of individual molecules, a key factor in understanding their behavior in various physical and chemical processes.
Unveiling the Properties of Compound F: Physical Attributes and Chemical Behaviors
In the fascinating world of chemistry, compound F stands out as a substance with intriguing physical and chemical properties. These characteristics are like a unique fingerprint that distinguishes it from other compounds and determines its behavior in various applications.
Physical Properties: A Matter of Form
Compound F boasts an array of physical properties that shape its behavior in different environments. Its melting point, the temperature at which it transitions from a solid to a liquid state, plays a crucial role in its processing and storage. Similarly, its boiling point, the temperature at which it transforms into a gas, influences its volatility and vapor pressure. Furthermore, its solubility in various solvents, such as water or organic solvents, affects its ability to dissolve and interact with other substances.
Chemical Properties: A Tale of Reactivity
Beyond its physical characteristics, compound F also exhibits distinct chemical properties. Its reactivity describes its eagerness to participate in chemical reactions. This property influences its use as a catalyst, a substance that accelerates reactions, or as a reagent, a substance that undergoes chemical transformations. Understanding its oxidation state, the charge associated with the atoms within the compound, is crucial for predicting its electron transfer capabilities and its potential to act as an oxidizing or reducing agent.
The Importance of Properties in Compound F Applications
The combination of physical and chemical properties in compound F makes it a versatile substance with wide-ranging applications. Its stability under certain conditions may allow for its use in harsh environments. Its flammability and explosiveness need to be carefully considered when handling and storing the compound. Moreover, its toxicity and environmental impact are important factors to assess when evaluating its potential applications.
Understanding the properties of compound F is akin to unraveling a hidden language. By deciphering its physical and chemical characteristics, scientists and researchers can harness its unique capabilities for various purposes. Whether in pharmaceuticals, manufacturing, or environmental science, the properties of compound F guide its use and ensure its safe and effective application.
Isomers: Unraveling the Hidden Variations
In the realm of chemistry, molecules can don different guises while maintaining the same molecular formula. These isomers mirror each other in composition but flaunt unique properties. Let’s explore the three main types of isomerism:
Structural Isomerism: Rearranging the Atoms
Structural isomers are like rearranged puzzles. They feature the same atoms but connected in different sequences. Each arrangement creates a distinct molecule with unique properties.
Take butane, for instance. It can exist as two structural isomers: n-butane (straight-chain) and isobutane (branched-chain). The former resembles a railroad track, while the latter resembles a fork in the road.
Stereoisomerism: Mirrored Images and More
Stereoisomers are mirror images of each other. They have the same atoms arranged in the same order, but differ in their spatial orientation. It’s like comparing your right hand to your left hand; they’re the same but not identical.
Enantiomers are non-superimposable mirror images, like two gloves. Diastereomers, on the other hand, are non-superimposable but not mirror images, like two different chairs.
Constitutional Isomerism: Different Connectivity
Constitutional isomers possess the same molecular formula but different connectivity of atoms. They resemble different molecules altogether.
For example, ethanol and dimethyl ether share the formula C2H6O. However, ethanol links its carbon atoms via a single bond, while dimethyl ether connects them via two bonds. This connectivity difference alters their properties and reactivity.
Understanding isomerism is crucial in chemistry, biochemistry, and the pharmaceutical industry. It helps predict molecular properties, explain biological activity, and guide drug design. So, next time you encounter a molecule, remember that its identity may not be as straightforward as it seems.
Synthesis: Crafting New Compounds
In the realm of chemistry, synthesis is an art form, allowing us to create new compounds from seemingly disparate elements. For Compound F, this process unfolds as a captivating tale of chemical transformations.
Organic Chemistry: The Foundation of Synthesis
Organic chemistry, the study of carbon-based compounds, forms the cornerstone of Compound F’s creation. It unveils the secrets of molecular structure, reactivity, and the ways in which molecules interact. Through this lens, we glimpse the intricate pathways that lead to Compound F’s genesis.
Reaction Mechanisms: The Story of Change
At the heart of synthesis lie reaction mechanisms, the detailed narratives of how chemical change unfolds. These mechanisms describe the steps involved in the transformation of reactants into products, providing insights into how Compound F is assembled molecule by molecule.
Catalysts: Agents of Orchestration
Like conductors leading an orchestra, catalysts play a crucial role in the symphony of Compound F’s synthesis. These substances, often themselves unchanged, accelerate reactions, guiding the transformation of reactants toward the desired outcome. Their presence ensures that the synthesis proceeds smoothly and efficiently.
With these elements in place, the synthesis of Compound F becomes a tapestry of chemical artistry, weaving together organic chemistry, reaction mechanisms, and catalysts to create a new compound that holds promise for a multitude of applications.
Applications: Compound F’s Versatile Presence in Diverse Industries
Compound F finds its prominence in a wide array of industries, ranging from culinary delights to lifesaving pharmaceuticals and beauty regimes.
Food Science: Preserving Delicacies
In food science, Compound F excels as a potent preservative, ensuring the longevity of our culinary creations. It restrains the growth of unwanted microorganisms, preserving the freshness and integrity of our cherished foods.
Pharmaceuticals: Healing and Alleviation
Compound F plays a crucial role in the pharmaceutical industry, where it contributes to the development of life-enhancing medications. It serves as an essential component in drugs that combat various ailments, from minor discomfort to life-threatening illnesses.
Cosmetics: Enhancing Beauty and Well-being
In the realm of cosmetics, Compound F infuses products with its remarkable abilities. It improves skin health, reduces signs of aging, and enhances overall aesthetic appeal. Its antioxidant properties safeguard against skin damage, while its moisturizing qualities nourish and soothe.
Toxicity: Understanding the Risks of Compound F
Exposure to Compound F:
From industrial settings to pharmaceutical products, Compound F has a wide range of applications. However, it’s crucial to understand its potential risks and how it can impact human health when exposure occurs.
Dose-Response Relationships:
Dose-response relationships reveal the connection between the amount of Compound F exposure and its effects. Low doses may result in minimal harm, while higher concentrations can lead to more severe consequences. Identifying these relationships helps establish safe exposure levels.
Hazard Assessment:
Hazard assessment evaluates the potential for Compound F to cause adverse effects. This involves considering factors such as its chemical structure, toxicity studies, and environmental persistence. Hazard assessment helps determine the likelihood and severity of potential risks.
Risk Management:
Based on hazard assessment findings, risk management strategies aim to minimize the chances of exposure and mitigate potential harm. This involves setting safety standards, implementing protective measures, and monitoring exposure levels. Effective risk management safeguards human health and the environment.
Additional Considerations:
In addition to the three key concepts above, several other factors contribute to understanding the toxicity of Compound F:
- Route of exposure: Inhalation, skin contact, or ingestion can affect the level of toxicity.
- Individual susceptibility: Age, health conditions, and genetics influence how people respond to exposure.
- Interactions with other substances: Exposure to multiple chemicals can have combined or synergistic effects.
Understanding the toxicity of Compound F is crucial to protect human health. By considering dose-response relationships, conducting hazard assessments, and implementing risk management strategies, we can minimize exposure and mitigate potential harm.
Metabolism: Tracing the Journey of Compound F Through the Body
Compound F embarks on a fascinating journey through the complex labyrinth of the human body, where it undergoes a series of intricate transformations. This process, known as metabolism, involves a multitude of chemical reactions that govern its absorption, distribution, and elimination.
Digestion: The Gateway to the Body
Compound F’s journey begins in the digestive system. Within the stomach’s acidic environment, it encounters enzymes that break down its molecular structure. As it traverses the small intestine, specialized villi absorb the resulting molecules, allowing them to enter the bloodstream.
Absorption: A Selective Passage
Once in the bloodstream, Compound F must navigate a gauntlet of barriers that determine its distribution throughout the body. Some compounds are rapidly absorbed, while others face restrictions. This process is influenced by factors such as the lipophilicity (fat-loving nature) and ionization state of Compound F.
Excretion: The Farewell Stage
The final chapter of Compound F’s journey is its elimination from the body. The kidneys play a crucial role in filtration, selectively removing waste products from the blood. Some compounds are also excreted through the liver, bile, or sweat. The rate and efficiency of excretion depend on various factors, including the half-life (time required for half of the compound to be removed) and the individual’s overall health.
Metabolism: A Balancing Act
Throughout its journey, Compound F interacts with various enzymes that catalyze its transformation. These enzymes can either activate or deactivate the compound, influencing its biological activity. The rates of these reactions are tightly regulated to maintain a delicate balance within the body.
The metabolism of Compound F is an intricate process that ensures its safe and effective absorption, distribution, and elimination. By understanding this journey, scientists can optimize drug delivery systems, predict potential side effects, and develop personalized treatments tailored to individual patient needs.
Biological Activity: Interacting with Life
In the realm of science, the study of biological activity delves into the fascinating interactions between compounds like Compound F and living organisms. This branch of chemistry unveils the intriguing ways in which compounds influence biochemical processes, regulate physiological functions, and interfere with disease states.
Pharmacology: Unraveling the Therapeutic Potential
Pharmacology centers on exploring the therapeutic properties of Compound F, investigating its ability to treat or prevent diseases. Scientists scrutinize how the compound interacts with target molecules in the body, triggering specific biochemical responses. These studies pave the way for the development of effective medications that can alleviate suffering and restore health.
Biochemistry: Unmasking Molecular Mechanisms
Biochemistry takes a closer look at the molecular mechanisms underlying Compound F’s biological activity. Researchers unravel the pathways through which the compound influences cellular processes, regulating gene expression, metabolic reactions, and signal transduction. This knowledge deepens our understanding of how Compound F exerts its effects and enables us to engineer compounds with tailored properties.
Drug Discovery: A Journey Towards Innovation
The insights gleaned from pharmacology and biochemistry fuel the exciting field of drug discovery. Scientists harness the biological activity of Compound F to design and develop new medications. This collaborative process involves screening, testing, and optimizing compounds to identify those with promising therapeutic potential. Ultimately, these discoveries lead to novel treatments that improve the lives of countless individuals.
Environmental Impact: Assessing the Consequences of Compound F
Understanding the environmental impact of chemical compounds is crucial for responsible stewardship of our planet. In the case of Compound F, we need to evaluate its potential effects on the environment throughout its life cycle.
Life Cycle Assessment: Tracking Compound F’s Journey
A life cycle assessment traces the environmental impact of a compound from its raw material extraction to its final disposal. For Compound F, this would involve considering the energy, water, and resources used in its production, as well as the emissions and waste generated at each stage.
Carbon Footprint: Measuring Greenhouse Gas Emissions
Compound F’s carbon footprint measures its contribution to greenhouse gas emissions. These gases trap heat in the atmosphere, contributing to climate change. By examining the fuel consumed, energy used, and emissions released during Compound F’s production and disposal, we can assess its role in global warming.
Ecotoxicity: Assessing Effects on Ecosystems
Ecotoxicity refers to the potential harm that Compound F poses to living organisms and ecosystems. Scientists conduct laboratory and field studies to evaluate the toxicity of Compound F to aquatic life, terrestrial animals, and soil organisms. Understanding its bioaccumulation and biomagnification potential is also crucial to assess its long-term effects on food chains.
By thoroughly assessing the environmental impact of Compound F, we can develop informed strategies to minimize its negative consequences. This may involve optimizing production processes, reducing emissions, and exploring sustainable disposal methods. Such efforts help us mitigate environmental risks and preserve our planet for future generations.