Competitive Enzyme Inhibitors: Mechanisms, Inhibition, And Impact On Enzyme Activity
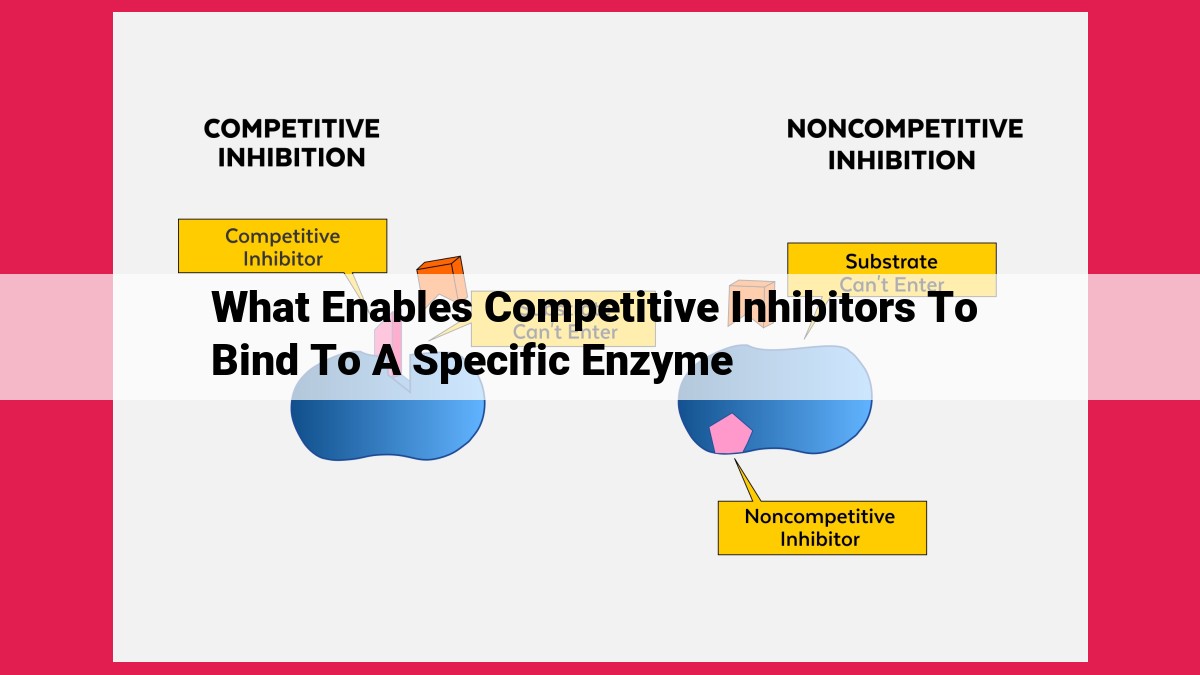
Competitive inhibitors effectively bind to enzymes by mimicking the substrate’s structure, enabling them to compete for the active site. These inhibitors occupy the enzyme’s binding site, preventing the substrate from accessing it. As a result, the enzyme’s catalytic activity is hindered, leading to reduced conversion of substrates into products. The binding affinity and selectivity of competitive inhibitors dictate their effectiveness in blocking enzyme activity.
Understanding Competitive Inhibition
- Definition and mechanism of competitive inhibition.
- Role of active site inhibition and how inhibitors prevent substrate binding.
- Substrate binding and how inhibitors compete for binding sites.
Understanding Competitive Inhibition: A Tale of Rival Binders
In the intricate world of biochemical reactions, enzymes reign supreme as the conductors of life’s processes. They orchestrate countless chemical transformations, enabling cells to thrive and function. However, sometimes, unwelcome guests known as inhibitors enter the picture, disrupting the harmony of enzyme catalysis.
The Enzyme’s Sanctuary: The Active Site
Enzymes possess a sacred chamber known as the active site, a meticulously designed niche where substrates, the molecules destined for transformation, meet their fate. With remarkable precision, the active site’s contours complement the substrate’s shape, creating an ideal environment for catalysis.
Substrate: The Enzyme’s Beloved Partner
Substrates are the lifeblood of enzymes, the molecules that eagerly bind to the active site and undergo transformation. Like a key fitting into a lock, substrates fit snugly into the active site, triggering a symphony of chemical events that lead to the desired products.
Inhibitors: The Unwelcome Intruders
Inhibitors, as their name suggests, are molecular adversaries that impede enzyme activity. They infiltrate the active site, vying for space with the rightful substrate. Competitive inhibitors, the focus of our tale, mimic the substrate’s structure, creating a rivalry for binding to the active site.
The Battle for the Active Site: A Struggle for Supremacy
As substrate and inhibitor compete for the active site, a fierce battle ensues. The strength of the binding interactions determines the outcome. Substrates, with their superior affinity for the active site, often emerge victorious, enabling enzyme catalysis to proceed uninterrupted. Inhibitors, however, can strike back, their high binding affinity displacing substrates and halting enzyme activity.
The Impact of Competitive Inhibition: A Ripple Effect
Competitive inhibition has far-reaching consequences, interfering with enzyme function and disrupting biochemical pathways. In the body, this can lead to a cascade of events, affecting metabolism, cell growth, and other vital processes.
Understanding Competitive Inhibition: A Key to Biomedical Advancements
By deciphering the intricacies of competitive inhibition, scientists can develop drugs that target specific enzymes, exploiting the molecular rivalry between inhibitors and substrates. These drugs can combat diseases, intervene in metabolic disorders, and alleviate a myriad of health conditions.
Competitive inhibition is a fascinating phenomenon in the realm of biochemistry, where rival molecules engage in a battle for supremacy over the enzyme’s active site. Understanding this process provides a powerful tool for scientists, enabling them to design drugs that manipulate enzyme activity and improve human health.
The Active Site: A Binding Hotspot
Enzymes, the workhorses of our bodies, are remarkable molecules that drive the essential chemical reactions that keep us alive. They do this through their active site, a small but highly specialized region where they bind to their target molecules, known as substrates. The active site is like a molecular lock and key, shaped precisely to accommodate specific substrates.
Substrate Recognition and Binding
When a substrate enters the active site, it is like finding the perfect fit. The substrate’s shape and chemical properties complement the active site’s geometry and charges. This precise fit allows the substrate to bind securely, forming enzyme-substrate complexes.
Competitive Inhibitors and Active Site Occupancy
Just as substrates bind to the active site, competitive inhibitors can also do so. These molecules mimic the structure of substrates, allowing them to compete for binding. When competitive inhibitors occupy the active site, they block the substrates from binding, effectively preventing enzyme activity.
This competition becomes a race for the active site, with the substrate and inhibitor vying for the same spot. The stronger the binding affinity of the inhibitor, the more likely it will outcompete the substrate and claim the active site, leading to potent enzyme inhibition.
Implications for Drug Design
Understanding the structure and function of the active site is crucial for drug design. By designing drugs that mimic the structures of substrates, researchers can develop potent and selective competitive inhibitors. These drugs can effectively block specific enzymes, thereby treating a wide range of diseases.
By delving into the molecular intricacies of the active site, we unlock the secrets of enzyme inhibition and open doors to new therapeutic possibilities.
Substrate: The Enzyme’s Chosen Partner
In the intricate dance of enzyme catalysis, substrates play a crucial role, guiding enzymes towards catalytic excellence. Substrates are molecules that enzymes specifically recognize and transform, acting as the raw materials for enzymatic reactions. They bind to the enzyme’s active site, a specialized region where catalysis occurs, with remarkable precision.
Enzyme specificity, a cornerstone of enzymatic function, ensures that the right substrate is selected, ensuring the correct chemical transformation. Enzymes achieve this selectivity through their unique structure, perfectly tailored to accommodate specific substrates. The active site, a molecular handshake, fits its designated substrate like a lock and key, forming an intricate bond that facilitates catalysis.
Competitive inhibitors, cunning impersonators, mimic the structure of substrates, thereby disrupting the enzyme-substrate union. These impostors compete with substrates for binding to the active site, effectively blocking the enzyme’s access to its true partner. As the concentration of competitive inhibitors increases, the enzyme’s catalytic efficiency dips, as the inhibitors outnumber the substrates, hindering the enzyme’s ability to perform its intended function.
Inhibitor: The Rival Binder
In the world of enzymes, where biochemical reactions dance delicately, inhibitors emerge as the enigmatic rivals, disrupting the harmonious flow of catalysis. These chemical interlopers bind to enzymes, wrestling for the active site—the enzyme’s sacred binding hotspot.
Mechanisms of Enzyme Inhibition
Inhibitors wield their power through diverse mechanisms. Competitive inhibitors engage in a direct battle with substrates, vying for the enzyme’s affection. By mimicking the structure of the substrate, they bind to the active site, effectively blocking the substrate’s access.
Non-competitive inhibitors take a more subtle approach. They do not compete directly with the substrate but bind to a different site on the enzyme, causing a conformational change. This change distorts the active site, rendering it unsuitable for substrate binding.
Uncompetitive inhibitors strike at a crucial moment—after the enzyme-substrate complex has formed. They bind to the enzyme-substrate complex, stabilizing it and preventing the reaction from proceeding.
Binding Affinity: The Strength of the Bond
The strength of an inhibitor’s grip on the enzyme is measured by its binding affinity. This affinity determines how potently the inhibitor inhibits the enzyme. The higher the binding affinity, the more effectively the inhibitor can block substrate binding and enzyme activity.
Selectivity and Target Specificity
Inhibitors are not indiscriminate foes. They exhibit selectivity, targeting specific enzymes or even specific isoforms of enzymes (isozyme inhibition). This selectivity is crucial for pharmacological applications, allowing inhibitors to target specific enzymes involved in diseases without affecting others.
The Power of Inhibitors
Inhibitors play a vital role in drug design and development. By selectively targeting enzymes responsible for diseases, they can effectively block their activity and restore biochemical balance. They are essential tools in the fight against infections, cancers, and a wide range of other ailments.
Enzyme-Inhibitor Interactions: The Binding Affinity Dance
In the captivating world of enzyme inhibition, binding affinity takes center stage. It’s the secret handshake between enzymes and inhibitors, determining their strength and selectivity. Enzymes, those remarkable molecular matchmakers, possess an active site, a sacred space shaped to perfectly cradle their substrates. Like a key fitting into a lock, substrates bind to the active site, triggering a flurry of catalytic reactions. But when an inhibitor steps into the picture, it’s like a mischievous interloper, mimicking the substrate and disrupting the enzyme’s dance with its true partner.
Inhibitor potency, another key player in this molecular drama, measures an inhibitor’s ability to bind to the enzyme. Think of it as a measure of the inhibitor’s persuasive powers. The higher the potency, the more likely the inhibitor is to snatch the enzyme’s attention away from the substrate. It’s like a siren song, luring the enzyme into its grasp.
But there’s more to the story than just binding strength. Selectivity is the inhibitor’s ability to distinguish between different enzymes. Some inhibitors are like promiscuous partygoers, binding to multiple enzymes. Others are more discerning, targeting specific enzymes like a skilled matchmaker. This selectivity can be crucial in drug development, where the goal is to inhibit a specific enzyme without disrupting the entire cellular symphony.
So, the dance between enzymes, inhibitors, and binding affinity is a delicate and intricate one. By understanding the interplay, we can design inhibitors with the right moves to target specific enzymes, paving the way for innovative therapies and targeted drug treatments.
Selectivity: The Enzyme’s Discrimination
- Enzyme specificity and how it allows discrimination between substrates.
- Isozyme inhibition and the targeting of specific enzyme forms.
- Enzyme family inhibition and the use of inhibitors to target multiple enzymes.
- Selective competitive inhibitors and their applications.
Selectivity: The Enzyme’s Discrimination
In the realm of enzymatic reactions, selectivity reigns supreme. Enzymes, the masterminds of biochemical transformations, possess an uncanny ability to differentiate between substrates, choosing their partners with meticulous precision.
Enzyme Specificity and Substrate Discernment
Enzymes are not indiscriminate. They harbor a remarkable specificity, recognizing and binding to only a select group of substrates. This ability stems from the enzyme’s active site, a tailored pocket that snugly accommodates specific substrates. Like a lock and key, the substrate’s shape and chemical properties must perfectly match the active site’s contours to foster a productive interaction.
Isozyme Inhibition: Targeting Specific Enzyme Forms
Within an enzyme family, isozymes are variants that share a common catalytic function but differ in their target substrates or regulatory properties. Competitive inhibitors can be employed to selectively inhibit specific isozymes, providing a means to modulate enzymatic activity in a targeted manner.
Enzyme Family Inhibition: Broadening the Scope of Inhibition
In some cases, competitive inhibitors can inhibit an entire enzyme family, rather than just a specific isozyme. These broad-spectrum inhibitors disrupt the activity of multiple related enzymes, offering a wider impact on cellular processes.
Selective Competitive Inhibitors: Applications in Research and Medicine
Selective competitive inhibitors are indispensable tools in both research and medicine. By precisely targeting specific enzymes or enzyme families, these inhibitors allow scientists to elucidate enzyme function, explore biochemical pathways, and develop targeted therapies for various diseases.
For example, the drug aspirin is a well-known selective competitive inhibitor of the enzyme cyclooxygenase-2 (COX-2). COX-2 is involved in inflammation, and aspirin’s inhibition of this enzyme reduces inflammation in the body, making it effective in treating conditions such as pain, fever, and arthritis.
In conclusion, the selectivity of enzymes is fundamental to their function and regulation. Competitive inhibitors harness this selectivity to target specific enzymes or enzyme families, providing valuable insights into enzyme biology and offering therapeutic options for a variety of diseases.