Cobalt: A Transition Metal With Versatile Chemical Properties
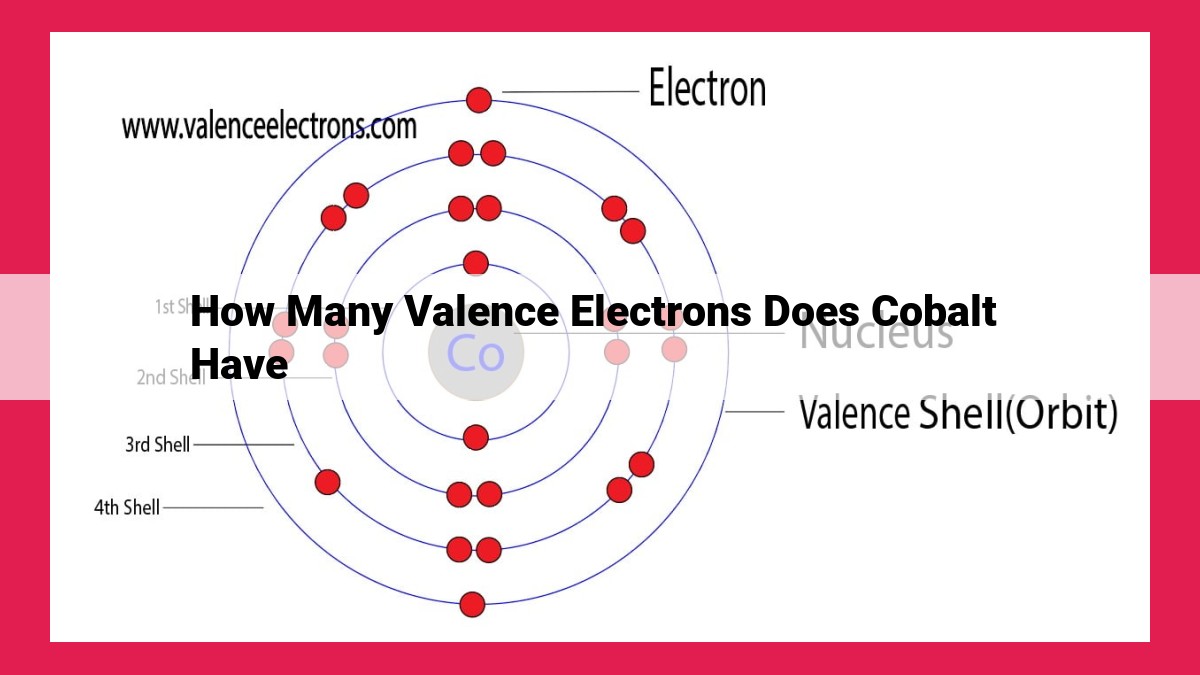
Cobalt, a transition metal, possesses a distinctive electron configuration, [Ar]3d74s2. Its valence electrons, located in the outermost orbitals (4s and 3d), play a crucial role in determining its chemical properties. As a d-block element, cobalt has the ability to exhibit variable oxidation states due to the presence of partially filled d orbitals. With 9 valence electrons, cobalt can participate in diverse chemical reactions and form numerous complexes, making it a versatile element in various industrial and scientific applications.
- Introduce cobalt as a transition metal and its importance.
- State the purpose of the blog post: to determine the number of valence electrons in cobalt.
** Cobalt: Unraveling the Mystery of Its Valence Electrons **
Cobalt, a gleaming transition metal, has captivated scientists and industries alike for centuries. Its unparalleled versatility in applications ranging from superalloys to medical devices stems from its unique electronic structure. In this blog post, we embark on a journey to determine the number of valence electrons in cobalt, unlocking the secrets behind its remarkable properties.
** Valence Electrons: The Key to Chemical Identity **
** Valence electrons** are the electrons occupying an atom’s outermost shell, playing a pivotal role in the chemical identity of an element. These electrons are instrumental in determining an element’s reactivity, bonding capabilities, and overall chemical behavior. Understanding the number of valence electrons in an element is crucial for comprehending its chemical properties.
Understanding Valence Electrons: A Journey into the World of Cobalt
In the realm of science, the world of transition metals holds a unique fascination. Among them, cobalt, a metal with a captivating bluish-gray hue, stands out for its versatility and importance. In this blog post, we embark on a journey to unveil the secrets of cobalt, specifically unraveling the mystery of its valence electrons.
Chapter 1: What are Valence Electrons?
Imagine an atom as a miniature solar system, with electrons orbiting its nucleus like celestial bodies. Valence electrons are the electrons residing in the outermost orbit of an atom, analogous to the planets orbiting the sun. They play a pivotal role in determining an element’s chemical behavior and reactivity.
Their Significance:
Valence electrons are the key players in the chemical dance of atoms. They determine an element’s ability to form chemical bonds, interact with other atoms, and create the diverse array of compounds we see in the world around us. Understanding their number and behavior is essential for comprehending the chemical properties of elements.
Determining the Number of Valence Electrons in Cobalt: A Journey into Atomic Structure
Valence electrons, those residing in the outermost shell of an atom, hold profound significance in shaping an element’s chemical identity. Understanding their number is crucial, especially for transition metals like cobalt. In this blog post, we embark on a captivating expedition to unravel the enigma of cobalt’s valence electrons, delving into the fascinating realm of atomic structure.
Cobalt: A Transition Metal of Versatility
Cobalt, a transition metal, resides in the periodic table’s fourth period and ninth group. This remarkable element plays a pivotal role in diverse industrial and scientific applications, including high-strength alloys, batteries, and even medical imaging. Its versatility stems from its unique electronic configuration, particularly the number of valence electrons it possesses.
Electron Configuration: A Window into Cobalt’s Atomic Arrangement
Electron configuration describes the distribution of electrons within an atom’s energy levels. For cobalt, its electron configuration is represented as [Ar]3d74s2. This notation signifies that cobalt possesses 29 electrons, arranged in the following manner:
- 2 electrons in the first energy level
- 8 electrons in the second energy level
- 18 electrons in the third energy level (filled as [Ar], representing argon)
- 1 electron in the fourth energy level
Valence Electrons: Unveiling Cobalt’s Reactive Nature
Valence electrons are those found in the outermost energy level, which, in cobalt’s case, are the 3d and 4s orbitals. These electrons determine an element’s chemical behavior and reactivity. Cobalt, with its 9 valence electrons (1 from 4s and 8 from 3d), exhibits a remarkable capacity for forming chemical bonds. This versatility makes cobalt an indispensable component in various alloys, catalysts, and pigments.
Implications of Cobalt’s Valence Electrons
Cobalt’s 9 valence electrons have far-reaching implications for its chemical properties. They enable cobalt to adopt multiple oxidation states, ranging from -2 to +6. This versatility allows cobalt to participate in a wide array of chemical reactions, making it a versatile player in industrial and scientific applications.
By exploring cobalt’s electron configuration, we have deciphered the mystery of its valence electrons. Cobalt’s 9 valence electrons, residing in the 3d and 4s orbitals, endow this transition metal with exceptional chemical versatility. This understanding paves the way for harnessing cobalt’s unique properties in a multitude of applications, from high-strength alloys to vital medical technologies.
Delving into the Realm of D-Block Elements: A Guide to Cobalt’s Chemical Character
D-Block Elements: A Unique Family of Chemistry
In the fascinating world of chemistry, elements are classified into distinct groups based on their electronic configurations. Among these groups, the d-block elements stand out with their distinctive properties. These elements are characterized by the presence of one or more electrons in their d orbitals, which are a set of five orbitals located in the outermost energy level of an atom. This unique electronic arrangement grants d-block elements remarkable chemical versatility.
Cobalt: A Versatile Transition Metal
One notable member of the d-block family is cobalt, represented by the symbol Co. As a transition metal, cobalt possesses a partially filled d orbital, which contributes to its diverse chemical behavior. The presence of partially filled d orbitals allows cobalt to exhibit a wide range of oxidation states, making it capable of forming stable compounds with differing oxidation numbers. This characteristic makes cobalt a valuable element in the field of chemistry, particularly in applications such as catalysis and battery technology.
Cobalt’s Electronic Configuration and Valence Electrons
To understand cobalt’s chemical properties, it’s essential to delve into its electronic configuration. The electron configuration of cobalt is [Ar]3d74s2, which indicates that it has seven electrons in its d orbitals and two electrons in its 4s orbital. The valence electrons of an element are those electrons that occupy the outermost energy level, and in the case of cobalt, it has nine valence electrons. These valence electrons play a crucial role in determining cobalt’s chemical reactivity and bonding properties.
Implications of Cobalt’s Valence Electrons
The number of valence electrons in cobalt significantly influences its chemical behavior. The presence of nine valence electrons makes cobalt capable of forming stable compounds with a wide range of elements. Cobalt’s ability to exhibit multiple oxidation states further enhances its versatility, allowing it to participate in various chemical reactions. These properties make cobalt a valuable element in industrial processes and catalytic applications.
Unlocking the Mystery of Cobalt: Unveiling the Number of Valence Electrons
In the realm of elements, cobalt shines as a pivotal transition metal, gracing our technological advancements and captivating the curiosity of scientists. This blog post embarks on a journey to unravel the enigma that surrounds cobalt’s valence electrons, the key to understanding its remarkable properties.
Valence Electrons: The Chemical Gateway
Valence electrons, those residing in the outermost shell of an atom, possess a profound influence on an element’s chemical behavior. They serve as the atomic ambassadors, venturing forth to forge bonds with atoms of other elements.
Electron Configuration: A Blueprint for Cobalt’s Electrons
Every element possesses a unique blueprint known as its electron configuration. For cobalt, this blueprint reads [Ar]3d74s2. This tells us that it has seven electrons in its 3d orbital and two electrons in its 4s orbital.
D-Block Elements: A Realm of Versatility
Cobalt proudly belongs to the d-block elements, a group characterized by the presence of electrons in the d orbital. These elements, including cobalt, exhibit remarkable versatility in bonding, enabling them to form a wide array of compounds with distinct properties.
Transition Metals: Masters of Disguise
Transition metals, a subset of the d-block elements, possess a partly filled d orbital. This unique configuration empowers them with the ability to change oxidation states, transforming their chemical identities and paving the way for the formation of complex compounds.
Cobalt’s Valence Electrons: A Symphony of Energy
Now, let’s unveil the number of valence electrons in cobalt. Delving into its electron configuration, we find nine valence electrons: seven from the 3d orbital and two from the 4s orbital. This combination of d and s electrons grants cobalt its extraordinary versatility, allowing it to participate in a myriad of chemical reactions.
Implications: Unraveling Cobalt’s Chemical Tapestry
The number of valence electrons in cobalt profoundly influences its chemical properties. It forms stable bonds with a diverse range of elements, making it an essential component in alloys, pigments, and batteries. Additionally, cobalt’s nine valence electrons enable it to form complexes with various ligands, expanding its applications in catalysis and medicine.
Cobalt’s Valence Electrons: A Journey into the Heart of a Transition Metal
Cobalt, a lustrous silvery-white metal, has a captivating story to tell, and at the heart of this story lie its valence electrons. These electrons are the gatekeepers of cobalt’s chemical personality, dictating its ability to form bonds, interact with other elements, and shape its remarkable properties.
Cobalt, a transition metal, resides in the d-block of the periodic table. This noble family of elements is characterized by their partly filled d orbitals. These orbitals, like tiny parking spaces for electrons, play a crucial role in cobalt’s electronic dance.
Electron Configuration: A Blueprint of Cobalt’s Electrons
To understand cobalt’s valence electrons, we need to unravel its electron configuration. This is the blueprint of an atom’s electron arrangement. For cobalt, the electron configuration reads: [Ar]3d74s2.
Breaking Down the Electron Configuration
Breaking down this blueprint, we see that:
- [Ar]: This represents argon’s electron configuration, indicating that cobalt has the same electron arrangement as argon up to this point.
- 3d7: This signifies that cobalt has seven electrons in its 3d orbital.
- 4s2: The final two electrons reside in the 4s orbital.
Determining Valence Electrons: The Key to Cobalt’s Chemistry
Valence electrons are the electrons in an atom’s outermost shell, the ones that actively participate in chemical reactions. In cobalt’s case, both the 4s and 3d orbitals contribute to its valence electrons.
Adding up the electrons in these orbitals, we find that cobalt has nine valence electrons. These nine electrons are the architects of cobalt’s chemical properties, determining its reactivity and the bonds it can form.
Implications of Valence Electrons: A Versatile Metal
Cobalt’s nine valence electrons give it an extraordinary versatility. This allows it to:
- Form a wide range of oxidation states, meaning it can gain or lose electrons readily.
- Participate in complex formation, where cobalt atoms bind to other molecules or ions, creating intricate structures.
Cobalt’s versatility makes it a key player in various industrial and technological applications, including alloys, batteries, and magnetic materials. Its unique electronic configuration enables it to take on diverse roles, making it an indispensable element in our modern world.
Understanding Cobalt: Determining Its Valence Electrons
Cobalt, a renowned transition metal, holds immense significance in scientific and industrial applications. Its unique properties, attributed to its electronic configuration, make it a fascinating element to explore. In this blog post, we delve into the captivating world of cobalt and unravel the mystery of its valence electrons.
Cobalt’s Electronic Structure: A Key to Understanding its Valence Electrons
Valence electrons, those residing in the outermost shell of an atom, play a pivotal role in determining an element’s chemical characteristics. Cobalt boasts an electron configuration of [Ar]3d74s2, indicating the presence of seven valence electrons. These valence electrons reside in two orbitals: the 3d orbital, which accommodates seven electrons, and the 4s orbital, where two electrons reside.
The Significance of Valence Electrons in Cobalt’s Properties
Cobalt’s array of valence electrons profoundly influences its chemical behavior. The presence of nine valence electrons endows cobalt with a fascinating versatility, allowing it to exhibit variable oxidation states and form numerous stable complexes. This versatility underscores cobalt’s pivotal role in diverse industrial and biological processes.
Cobalt’s Versatility: A Multifaceted Element
Cobalt’s remarkable versatility stems from its exceptional ability to engage in various chemical reactions. Its nine valence electrons enable it to interact with a wide range of ligands, forming intricate coordination complexes. This characteristic makes cobalt a versatile catalyst in numerous industrial applications. Additionally, cobalt’s multifaceted nature extends to its biological functions, where it serves as an essential component of vitamin B12 and plays a crucial role in oxygen transport and metabolism.
Cobalt’s valence electrons stand as the cornerstone of its exceptional properties, shaping its chemical behavior and endowing it with remarkable versatility. Understanding the significance of valence electrons provides a profound insight into the intricate world of chemistry and the boundless possibilities it holds.