Chromium: An Element With Atomic Number 24
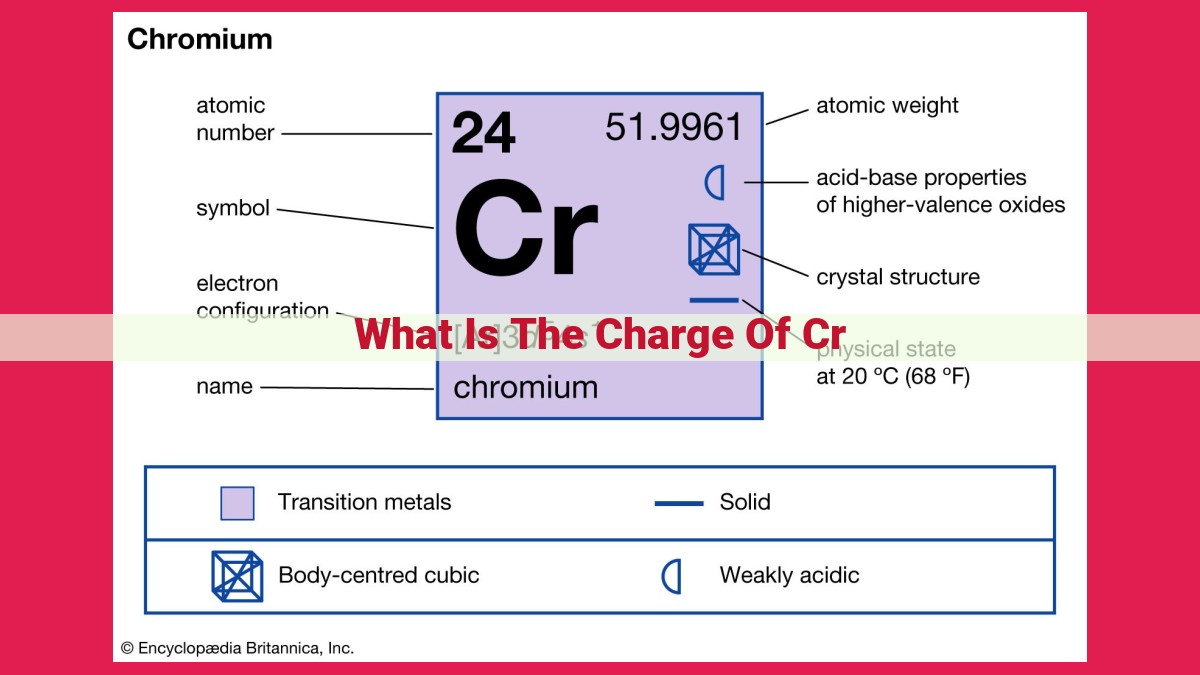
Chromium (Cr) is an element with an atomic number of 24, meaning it has 24 protons in its nucleus. The number of protons determines an element’s identity. In a neutral atom, the number of protons is equal to the number of electrons, resulting in a balanced charge. However, when an atom loses or gains electrons, it becomes an ion, which carries an electrical charge. The charge of an ion depends on the number of electrons lost or gained.
Delving into the Heart of Matter: Understanding Atomic Structure
Atomic Number: The Fingerprint of an Element
Every atom, the fundamental building block of matter, carries a unique identity number known as its atomic number. This number, denoted by Z, represents the number of protons within the atom’s nucleus. Protons, heavy and positively charged particles, define an element’s chemical properties and distinguish it from all others. For instance, all atoms with an atomic number of 1 are hydrogen, while those with an atomic number of 6 are carbon.
Atomic Mass: Weighing the Atom
Alongside its atomic number, each atom has an atomic mass, denoted by A. Atomic mass measures the atom’s weight, considering both protons and neutrons in its nucleus. However, the contribution of electrons, with their negligible mass, is generally disregarded.
Isotopes: Variations within an Element
Atoms of the same element can exist in different mass variations, called isotopes. Isotopes share the same atomic number, indicating an equivalent number of protons. However, they differ in the number of neutrons, resulting in different atomic masses. For example, carbon exists as three stable isotopes: carbon-12, carbon-13, and carbon-14.
Nuclides: A Precise Atomic Description
The complete identification of an atom requires both its atomic number and atomic mass, collectively known as its nuclide. Nuclides are unique representations of specific atomic species, providing an exact description of their nuclear composition.
Electronic Charge and Electrical Properties
In the realm of chemistry and physics, electronic charge plays a pivotal role in shaping the electrical behavior of matter. This fundamental quantity, denoted by the symbol e, represents the charge carried by an electron, the negatively charged subatomic particle that orbits the nucleus of an atom.
Electronic charge is a key player in the electrostatic force of attraction or repulsion between charged particles, as described by Coulomb’s law. This law quantifies the strength of the electrostatic force between two charged objects, based on their charges and the distance between them.
The electric potential of a charge distribution is another critical concept in understanding electrical properties. It is a scalar quantity that describes the amount of electrical work needed to move a unit positive charge from a reference point to a specific location in the field.
Electronic charge also lies at the heart of electric current, the flow of charged particles. When charged particles, such as electrons or ions, move through a conductor, they constitute an electric current. The magnitude of the current is directly proportional to the number of charged particles flowing per unit time and the charge carried by each particle.
Understanding electronic charge and its role in electrical properties is essential for comprehending various physical phenomena and technological applications, ranging from the behavior of electrical circuits to the operation of electronic devices and the chemistry of ionic compounds.
Formation of Ions: The Electrifying World of Charged Particles
In the realm of chemistry, ions are fascinating entities that bring atoms and molecules to life with their electric charge. These charged particles play a pivotal role in shaping the building blocks of matter and driving chemical reactions.
Introducing Ions: Atoms with a Twist
An ion is simply an atom or molecule that has lost or gained electrons, resulting in a net electric charge. When an atom loses electrons, it becomes positively charged and is known as a cation. Conversely, when an atom gains electrons, it acquires a negative charge and is called an anion.
The Dance of Cations and Anions: Ionic Bonding
The attraction between oppositely charged ions forms ionic bonds, the fundamental force that holds salts together. Consider sodium (Na) and chlorine (Cl) atoms. Sodium readily gives up an electron, leaving behind a positively charged Na+ ion. Chlorine, on the other hand, eagerly accepts this electron, transforming into a negatively charged Cl- ion. The electrostatic attraction between Na+ and Cl- ions forms sodium chloride (NaCl), the common table salt we use every day.
The Importance of Valence Electrons
The valence electrons, the electrons in the outermost energy level of an atom, play a crucial role in ion formation. Atoms strive to achieve a stable electron configuration, typically with eight valence electrons. Sodium has one valence electron, which it readily gives up to achieve this stability. Chlorine, with seven valence electrons, needs one more to complete its outer shell, which it gains by accepting an electron.
Understanding Oxidation Numbers: The Key to Ionic Charge
The oxidation number of an atom indicates the number of electrons it has lost or gained. For example, Na has an oxidation number of +1 in NaCl, while Cl has an oxidation number of -1. Oxidation numbers help us predict the charges of ions and understand the chemical reactions they undergo.
Closing Thoughts: The Charge of Life
Ions are not just abstract concepts; they are the very building blocks of the world around us. From the salts we use to season our food to the electrolytes that regulate our bodies, ions play an indispensable role in our daily lives. Understanding their formation and properties opens up a fascinating chapter in the world of chemistry, revealing the hidden dance of charged particles.
Valence Electrons: The Key to Understanding Chemical Reactivity
Imagine atoms as tiny building blocks, each with a unique set of electrons circling their nucleus. These valence electrons, located in the outermost energy level, play a pivotal role in determining an atom’s chemical behavior.
Valence electrons are like social butterflies, eagerly seeking interactions with electrons from other atoms. They form the basis of chemical bonds, the glue that holds atoms together to create molecules and compounds. The number of valence electrons an atom possesses determines its reactivity, the tendency to engage in chemical reactions.
Oxidation number is a valuable concept in understanding ionic charge. It represents the hypothetical charge an atom would have if it lost or gained all of its valence electrons. This concept is crucial in predicting the formation of ions, charged particles that form the backbone of ionic compounds such as sodium chloride (NaCl).
Finally, electron configuration provides insights into an atom’s reactivity. The arrangement of electrons within energy levels influences the atom’s stability and its ability to participate in chemical reactions. For instance, atoms with a full valence shell are less reactive, while those with an incomplete valence shell are more eager to interact with other atoms.
Understanding valence electrons is like unlocking the secrets of chemical reactivity. It empowers us to predict the formation of compounds, unravel the nature of ionic bonds, and decipher the intricate symphony of electrons that governs our world.