Understanding Chromatograms: A Comprehensive Guide To Peak Analysis And Interpretation For Analytical Chemistry
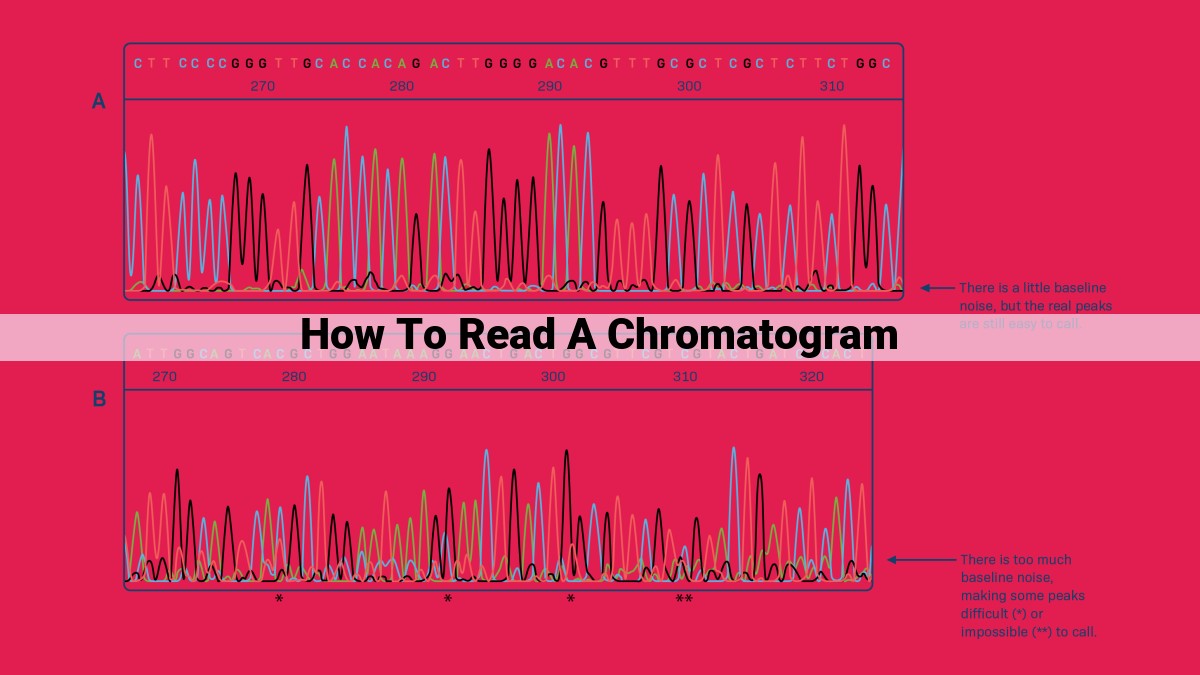
Reading a chromatogram involves identifying the baseline, locating peaks, and interpreting their characteristics. Peak retention time relates to compound properties, peak area reflects concentration, and peak height can estimate concentration. Noise, signal-to-noise ratio, resolution, column efficiency, and selectivity impact separation. Steps include identifying baseline, locating peaks, calculating retention time, and estimating peak area. Chromatograms are used in various fields to identify compounds, determine concentrations, and solve analytical problems.
Chromatography: The Alchemist’s Tool for Unraveling the Secrets of Mixtures
In the world of science and analysis, chromatography stands as a technique as elegant as it is powerful. It’s akin to an analytical alchemist, transforming complex mixtures into telltale patterns that reveal the identity and quantity of their individual components.
Imagine a fluid eluent flowing steadily through a stationary phase, which might be a packed bed or a coated surface. When a mixture of compounds is injected into this system, its constituents embark on a molecular dance, their fates determined by their nature. Larger, less polar molecules prefer to dance with the stationary phase, while smaller, more polar molecules cling closer to the eluent.
As the dance unfolds, the molecules face a constant dilemma: whether to embrace the stationary phase or surrender to the eluent’s pull. This dance, in conjunction with the precise control of flow rate, pressure, and other variables, separates the components based on their distinct affinities, allowing them to emerge from the system in a predictable sequence.
Understanding Chromatogram Basics
In the realm of analytical chemistry, chromatography stands as a powerful technique for separating and identifying compounds. At its core lies the concept of a chromatogram, a graphical representation of the separation process. To decipher these chromatograms, we must first grasp their fundamental components:
Key Chromatogram Parameters
-
Retention Time: This time indicates how long it takes for a compound to travel through the chromatographic system. It serves as a unique identifier for each compound under a specific set of conditions.
-
Peak Area: The area enclosed by the peak on the chromatogram is directly proportional to the concentration of the corresponding compound in the sample. Larger peak areas indicate higher concentrations.
-
Peak Height: The vertical distance from the baseline (the line connecting the base of the peaks) to the apex of the peak provides an estimate of the compound’s concentration.
Peak Retention Influencers
The retention time of a compound is influenced by multiple factors, including:
-
Molecular Weight: Generally, larger molecules have longer retention times as they interact more strongly with the stationary phase of the chromatographic system.
-
Polarity: Polar compounds tend to have longer retention times than nonpolar compounds due to their stronger interactions with the polar stationary phase.
-
Column Interactions: The nature of the stationary phase can significantly affect retention times. Different stationary phases have varying affinities for different compounds, leading to changes in their relative retention times.
Understanding these parameters and their influences is crucial for interpreting chromatograms accurately. In the next section, we will delve into the interpretation process, providing a step-by-step guide to unlock the wealth of information contained within these graphical representations.
Exploring Chromatographic Concepts
In the realm of chromatography, a powerful analytical technique, we delve into the depths of understanding chromatographic concepts that are key to unlocking the secrets of separation and identification.
Peak Area and Height as Measures of Concentration
The peak area plays a crucial role as a direct measure of the concentration of a given analyte. This area represents the amount of analyte present in the sample. On the other hand, the peak height provides an indirect measure of concentration, making it useful for concentration estimation.
Signal-to-Noise Ratio and Resolution
Noise, an ever-present challenge in analytical measurements, refers to random fluctuations that interfere with the signal of interest. The signal-to-noise ratio (S/N) quantifies the quality of a signal by comparing its strength to the surrounding noise. A high S/N ratio is desirable, indicating a clear signal.
Resolution, a crucial parameter in chromatography, measures the separation between two peaks. High resolution is essential for accurate identification and quantification of analytes. It is influenced by factors such as column efficiency and column selectivity.
Column Efficiency and Selectivity
Column efficiency refers to the ability of a column to separate compounds effectively. It depends on the number of theoretical plates in the column, which is directly proportional to the length of the column and inversely proportional to particle size.
Column selectivity refers to the ability of a column to differentiate between different types of compounds. It depends on the interactions between the compounds and the stationary phase of the column. A high selectivity column can separate compounds with similar chemical properties.
Mastering Chromatographic Concepts
Through a deeper understanding of peak area, peak height, signal-to-noise ratio, resolution, column efficiency, and column selectivity, we unlock the full potential of chromatogram interpretation. These concepts enable us to extract valuable information from chromatograms, unlocking the secrets of complex samples with greater accuracy and confidence.
Interpreting a Chromatogram: A Step-by-Step Guide
Chromatography is a powerful analytical technique used to separate and identify compounds. A chromatogram is the graphical representation of the separation, with the peaks corresponding to the different compounds.
To interpret a chromatogram, follow these steps:
- Identify the baseline. This is the horizontal line at the bottom of the chromatogram. It represents the detector’s response when no sample is present.
- Locate the peaks. Peaks are upward deviations from the baseline. Each peak represents a different compound.
- Determine the retention time. This is the time it takes for a compound to travel from the injection point to the detector. It is measured from the point of injection to the peak’s apex.
- Calculate the peak area. This is the area under the peak. It is proportional to the concentration of the compound.
- Evaluate the peak shape. The shape of a peak can provide information about the compound’s properties. For example, a symmetrical peak indicates a pure compound, while an asymmetrical peak may indicate the presence of impurities.
Here is an example of how to interpret a chromatogram:
[Image of a chromatogram with three peaks]
The chromatogram has three peaks. The first peak has a retention time of 5 minutes, a peak area of 100, and a symmetrical shape. The second peak has a retention time of 10 minutes, a peak area of 50, and an asymmetrical shape. The third peak has a retention time of 15 minutes, a peak area of 25, and a symmetrical shape.
Based on this information, we can infer that:
- The compound corresponding to the first peak is the most concentrated.
- The compound corresponding to the second peak is less concentrated and may contain impurities.
- The compound corresponding to the third peak is the least concentrated.
By following these steps, you can interpret chromatograms and use them to identify and quantify compounds.
Applications of Chromatogram Interpretation: Unleashing its Power in Diverse Fields
Chromatography’s versatility extends far beyond mere separation and identification of compounds. Its applications permeate a wide range of scientific disciplines, each harnessing its power to address unique challenges.
Analytical Chemistry: Unveiling the Secrets of Matter
In analytical chemistry, chromatograms serve as windows into the composition of substances. Precise peak identification and quantification allow chemists to determine the concentration, purity, and structural characteristics of molecules in complex samples. From pharmaceutical formulations to environmental samples, chromatograms provide critical insights for quality control, research, and forensic analysis.
Pharmaceuticals: Ensuring Drug Safety and Efficacy
Chromatography plays a pivotal role in the development and quality assurance of pharmaceuticals. Chromatograms enable precise monitoring of drug substances, impurities, and degradation products throughout the manufacturing process. Accurate interpretation of these patterns ensures the safety, efficacy, and regulatory compliance of drugs that touch the lives of millions.
Environmental Science: Guardians of the Earth
Chromatograms provide invaluable data for understanding and protecting our environment. By identifying and quantifying pollutants in air, water, and soil samples, scientists can assess contamination levels, trace the movement of chemicals through ecosystems, and develop strategies for remediation. Accurate chromatogram interpretation is crucial for environmental monitoring, risk assessment, and the preservation of our planet.
Importance of Accurate Interpretation: The Key to Informed Decisions
The ability to interpret chromatograms accurately is paramount for making informed decisions based on the information they contain. Correct identification and quantification of peaks ensure reliable results, while understanding peak shape, resolution, and noise levels allows for a robust assessment of data quality. Misinterpretation of chromatograms can lead to erroneous conclusions and potentially harmful consequences.
Therefore, mastering chromatogram interpretation is essential for scientists, researchers, and analysts in diverse fields. By honing their skills in this area, they unlock the full potential of chromatography, enabling them to unravel the secrets of matter, ensure the safety of pharmaceuticals, and protect our environment.