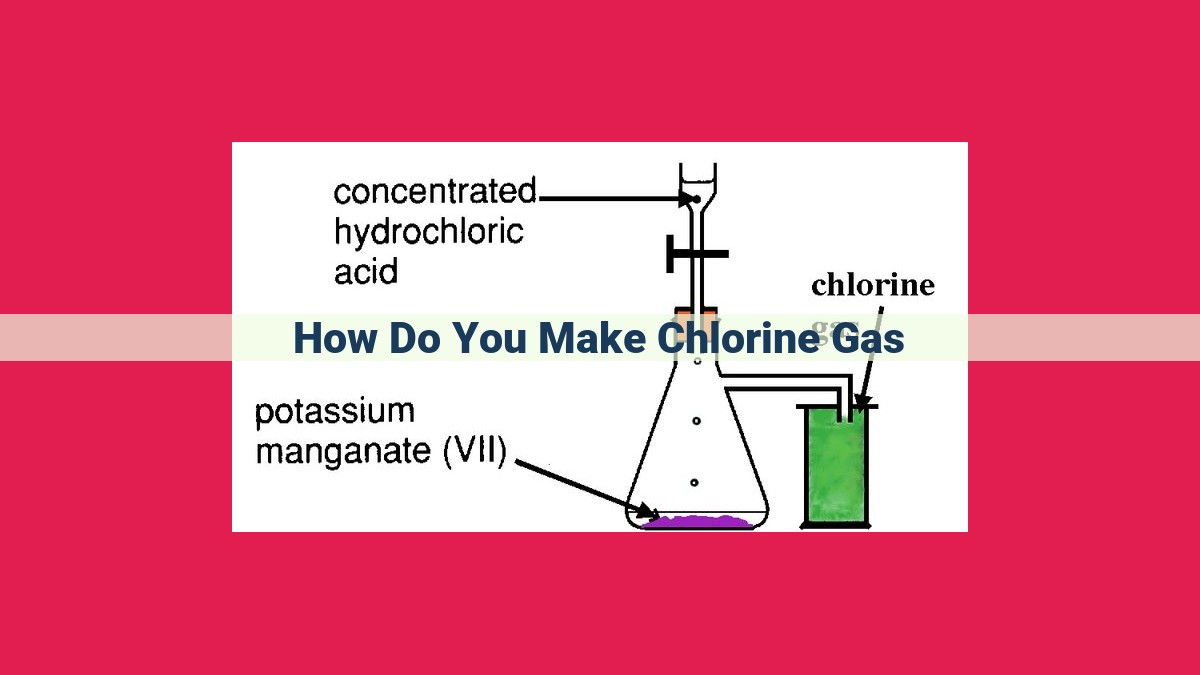
Chlorine Gas Production: Electrolysis For Water Purification, Bleaching &Amp; Industrial Applications
Electrolysis, involving the passage of an electric current through a salt solution (sodium chloride), enables the production of chlorine gas. During electrolysis, oxidation occurs at the anode, resulting in the formation of chlorine gas (Cl2). Hydrogen gas (H2) is simultaneously produced at the cathode. A salt bridge connects the anode and cathode solutions, completing the electrical circuit. The chlorine gas bubbles rise to the surface, allowing for collection. This process finds extensive applications in water purification, bleaching, and various industrial sectors.
Understanding Electrolysis
- Definition of electrolysis
- Role of positive and negative electrodes
Electrolysis: Unlocking the Secrets of Chemical Change
In the realm of chemistry, electrolysis emerges as a fascinating process that involves the transformation of substances through the power of electricity. Imagine a conductor immersed in a solution, creating a circuit that sets the stage for a remarkable chemical dance.
Electrolysis, in its essence, is the decomposition of a compound using an electric current. It’s like a magical wand that can break apart the molecules of a substance, rearranging their atoms to create new and useful products.
Positive and Negative Electrodes: The Guiding Forces
At the heart of electrolysis lie two crucial players: the positive electrode (anode) and the negative electrode (cathode). These electrodes act as gateways for the flow of electricity, directing the charged particles that drive the chemical reactions.
When the current flows, positive ions (cations) are drawn to the cathode, while negative ions (anions) flock to the anode. It’s as if the electrodes possess a magnetic pull, guiding the ions towards their respective destinations.
Oxidation and Reduction: A Balancing Act
As the ions interact with the electrodes, they undergo intriguing chemical transformations. Oxidation occurs at the anode, where electrons are stripped away from the ions, causing them to lose their negative charge. Conversely, at the cathode, reduction takes place, as the ions gain electrons and become more negatively charged.
In the production of chlorine gas, for example, the anode becomes the site of oxidation, where chloride ions lose electrons to form chlorine atoms. These atoms then combine to create the pungent, greenish gas that we recognize as chlorine.
Oxidation and Reduction: The Key Players in Electrolysis
Electrolysis, the process of breaking down compounds using electricity, involves two crucial electrochemical reactions: oxidation and reduction. Oxidation occurs when a substance loses electrons, while reduction involves gaining electrons.
In the electrolysis of sodium chloride (NaCl) to produce chlorine gas, the anode, the positively charged electrode, serves as the site of oxidation. At the anode, sodium (Na) atoms in the NaCl lose electrons and become positively charged sodium ions (Na+). These Na+ ions are then attracted to the negatively charged cathode.
Simultaneously, at the cathode, reduction occurs. Water molecules (H2O) gain electrons and undergo a transformation. They split into hydrogen gas (H2), which collects as bubbles on the cathode, and hydroxide ions (OH-).
The electrons that sodium atoms give up during oxidation flow through an external circuit to the cathode, where they participate in the reduction of water molecules. This electron transfer completes the electrical circuit necessary for electrolysis to occur.
By understanding the roles of oxidation and reduction, we gain a deeper insight into the intricate process of electrolysis and its applications in industries such as water purification and bleaching.
The Role of the Salt Bridge
- Explanation of the function of a salt bridge in completing the electrical circuit
The Magic of the Salt Bridge: A Lifeline for Electrolytic Reactions
In the realm of electrolysis, where chemical reactions dance to the rhythm of electricity, there is an unsung hero that plays a crucial role: the salt bridge. Its humble presence may be easily overlooked, but its function is indispensable.
Imagine a grand ballroom where the electrodes, the charged plates that attract and repel ions, are the stars of the show. Positive ions, like eager dancers, throng around the cathode, the negatively charged electrode, while their negative counterparts flock to the anode, the positively charged electrode. However, without a way for these ions to gracefully navigate between the two, the party would quickly grind to a halt.
Enter the salt bridge, the maestro that orchestrates this ionic tango. It is a simple yet brilliant device, a tube filled with a solution of ions, often sodium chloride (NaCl). These ions, like tiny chaperones, escort the charged particles from one electrode to the other, completing the electrical circuit. Without this crucial connection, the ions would become stranded, and electrolysis would be but a distant dream.
The salt bridge not only ensures the smooth flow of ions but also maintains electrical neutrality, preventing the accumulation of charges that would disrupt the delicate dance of electrolysis. It bridges the gap between the two half-reactions, allowing the electrons to dance their way through the external circuit, while the ions and molecules waltz within the solution.
In essence, the salt bridge is the lifeline of any electrolytic reaction. It plays a pivotal role in the production of essential chemicals like chlorine gas, the disinfectant that safeguards our water and keeps our homes sanitized. Without this humble device, the world of electrolysis would be a chaotic symphony of disjointed reactions.
Electrolysis of Sodium Chloride: A Story of Chemistry in Action
In the realm of chemistry, electrolysis holds a captivating power. This process harnesses the energy of electricity to drive chemical reactions, opening up a vast array of possibilities. One such application is the electrolysis of sodium chloride, a process that lies at the heart of chlorine gas production.
Picture a beaker filled with a sodium chloride solution, two electrodes immersed within it. As an electric current flows through the solution, a mesmerizing transformation unfolds. The positive electrode (anode) becomes a stage for oxidation, a reaction where electrons are stripped away from sodium ions, leaving behind positively charged chlorine ions. These ions, eager to reunite with their lost electrons, flock towards the negative electrode (cathode).
At the cathode, the dance continues with reduction, a reaction where electrons are generously donated to hydrogen ions. These ions, their thirst quenched, transform into hydrogen gas bubbles that rise to the surface, their presence a testament to the chemical artistry taking place.
Meanwhile, the chlorine ions that escaped the clutches of the anode gather at the surface of the solution, forming menacing chlorine gas bubbles. These bubbles, imbued with their potent chemical properties, await release into the world.
Through this intricate interplay of oxidation and reduction, electrolysis of sodium chloride yields both chlorine gas and hydrogen gas. These products have found widespread applications across industries. Chlorine gas, a potent disinfectant, plays a crucial role in water purification and bleaching processes, safeguarding public health and enhancing the quality of our lives.
The Versatile Chlorine Gas: Applications that Enhance Our Lives
Chlorine Gas: The Unsung Hero of Modern Society
In the realm of chemistry, electrolysis holds a pivotal role in unlocking the potential of elements and compounds. Electrolysis of sodium chloride, in particular, yields chlorine gas, an incredibly versatile substance with a wide range of applications that touch and enhance our daily lives.
Unveiling the Wonders of Chlorine Gas
Chlorine gas plays a crucial role in various industries, including:
-
Water Purification: Chlorine is the backbone of modern drinking water treatment systems, effectively eliminating harmful pathogens that can cause waterborne diseases. Its disinfecting properties ensure the safety of our tap water, protecting us from harmful contaminants.
-
Bleaching: The textile industry heavily relies on chlorine gas to whiten and brighten fabrics. By oxidizing dyes, chlorine removes unwanted colors, resulting in the pristine whites we see in sheets, towels, and clothing.
-
Paper Production: Chlorine gas is employed to bleach paper pulp, producing the bright, white paper we use for writing, printing, and packaging. It enhances the quality and aesthetics of paper products, making them more suitable for a variety of applications.
-
Chemical Synthesis: Chlorine gas serves as a fundamental building block in the production of numerous chemicals, such as PVC plastics, solvents, and pesticides. These chemicals find widespread use in construction, industrial processes, and agriculture.
Chlorine’s Impact on Our Living Standards
Beyond its industrial applications, chlorine gas also plays a crucial role in:
-
Medical Disinfection: Chlorine-based disinfectants are essential in healthcare settings, effectively killing bacteria and viruses on surfaces, medical devices, and in water systems. They ensure sanitary conditions, reducing the spread of infections and improving patient safety.
-
Swimming Pool Sanitation: Chlorine is widely used to sanitize swimming pools and hot tubs, protecting swimmers from waterborne pathogens. It maintains clean, safe, and refreshing water for recreational activities and relaxation.
-
Air and Odor Control: Chlorine gas is employed in air and odor control systems, neutralizing unpleasant smells and purifying the air in industrial settings, public spaces, and wastewater treatment plants.
Chlorine gas, although often overlooked, is an indispensable element in our society. Its unique properties have enabled a wide range of applications, enhancing our health, industries, and living standards. From disinfecting our water to whitening our fabrics and safeguarding our health in countless ways, chlorine gas plays a vital role in shaping our modern world.