Uncover The Chemical Significance Of Sodium: An Essential Element With A Unique Electron Configuration
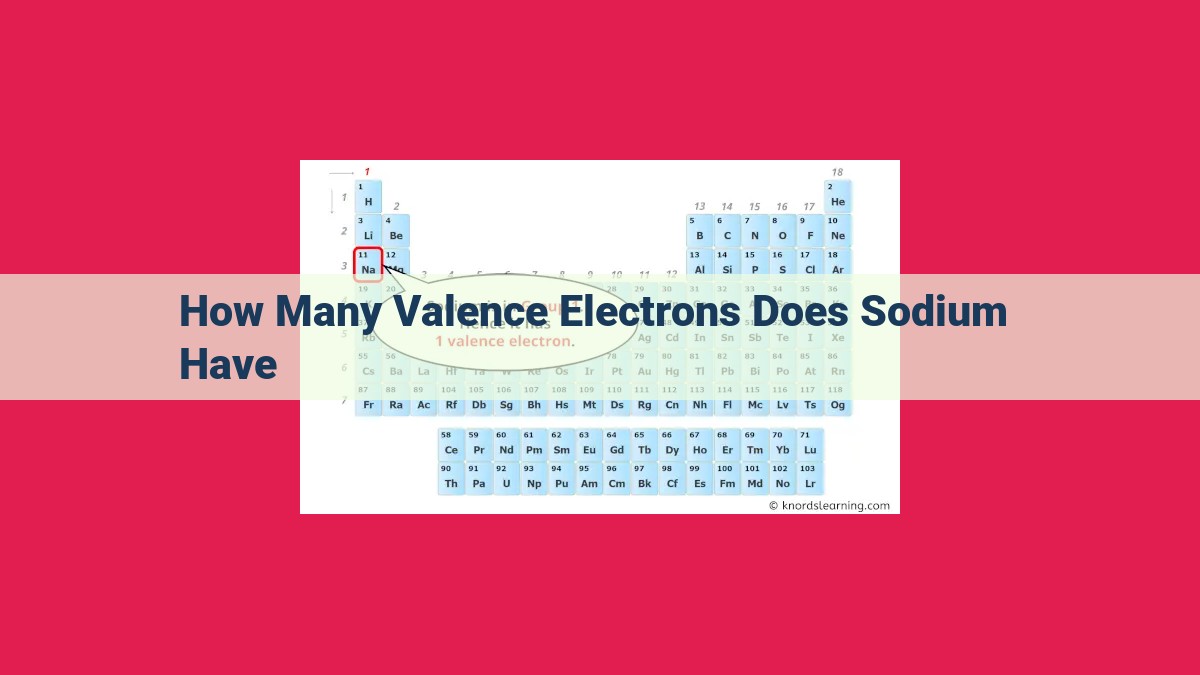
Sodium, an essential element, has a unique electronic structure that determines its chemical properties. With an atomic number of 11, it has 11 electrons. The electron configuration of sodium [Ne]3s¹ indicates that it has a single electron in its outermost shell, the valence shell. This lone valence electron makes sodium highly reactive and a powerful reducing agent. Its tendency to lose this electron leads to the formation of ionic bonds, contributing to its role in various chemical reactions and physiological processes. Understanding the number of valence electrons in sodium is crucial for comprehending its chemical behavior and its significance in numerous applications.
- Introduce the importance of understanding the number of valence electrons in an element, specifically sodium.
- Briefly explain the concept of valence electrons.
Unlocking the Secrets of Sodium’s Valence Electrons
In the realm of chemistry, understanding the behavior of elements is crucial. Sodium, a highly reactive metal, holds a special place in this puzzle due to its unique number of valence electrons. Join us on an exploration to unravel the fascinating world of sodium’s valence electrons, and witness how they shape its chemical personality.
What are Valence Electrons?
Imagine atoms as tiny solar systems, with a nucleus at their core and electrons whizzing around it like planets. Valence electrons are the electrons occupying the outermost orbit of an atom. Think of them as the social butterflies of the atomic realm, eager to interact with other atoms and molecules. The number of valence electrons profoundly influences an element’s chemical properties.
The Atomic Number of Sodium: A Key to Understanding its Properties
In the realm of chemistry, understanding the number of valence electrons in an element is crucial for unraveling its chemical behavior. One element that stands out in this regard is sodium, a highly reactive alkali metal. Its atomic number holds the key to unlocking the mysteries surrounding its valence electrons.
What is Atomic Number?
Think of the atomic number as the unique identity card of an element. It represents the number of positively charged protons found in the nucleus of an atom. This number defines the element and governs many of its properties, including its chemical behavior.
Sodium’s Atomic Number
Sodium, with an atomic number of 11, proudly boasts 11 protons in its nucleus. This atomic number has profound implications for the number of electrons orbiting its nucleus.
The Significance of 11
The number 11 dictates the number of electrons in a neutral sodium atom. Neutrality means that the total number of negatively charged electrons must equal the number of positively charged protons. Therefore, sodium has 11 electrons, balancing out the 11 protons in its nucleus.
Understanding sodium’s atomic number and its impact on the number of electrons is a fundamental step in comprehending its chemical nature. Armed with this knowledge, we can delve deeper into its electron configuration and uncover the secrets behind its exceptional reactivity.
The Electron Configuration of Sodium: A Chemical Storytelling
In the realm of chemistry, understanding the arrangement of electrons within an atom is crucial for deciphering its properties and behavior. This concept is known as electron configuration, and it plays a pivotal role in unraveling the secrets of sodium, a highly reactive element that has captivated scientists for centuries.
Sodium’s atomic number, which determines its unique identity among the elements, is 11. This number not only provides a fingerprint for sodium but also holds the key to its electron configuration. To comprehend sodium’s electron configuration, we must delve into the intricacies of atomic structure.
An atom consists of a nucleus, the dense core composed of protons and neutrons, surrounded by a cloud of electrons. These electrons occupy specific energy levels, forming distinct orbitals. The outermost energy level, known as the valence shell, houses the valence electrons, which are the most influential in determining an element’s chemical properties.
Sodium’s electron configuration is denoted as 1s² 2s² 2p⁶ 3s¹. This notation indicates that sodium has three energy levels, with two electrons in the first energy level (1s²), two in the second energy level (2s²), and six in the third energy level (2p⁶). Crucially, sodium has a single electron in its outermost energy level (3s¹).
This lone valence electron is what makes sodium so chemically active. Valence electrons are the electrons that participate in chemical bonding, forming connections with other atoms to create molecules. Sodium’s single valence electron eagerly seeks out partners, making sodium a good reducing agent and highly reactive with other elements.
By understanding sodium’s electron configuration, we gain insights into its chemical behavior. The lone valence electron is responsible for sodium’s reactivity, influencing its ability to form chemical bonds and interact with its surroundings. This knowledge is essential for unraveling the mysteries of sodium and unlocking its potential in various chemical applications.
Valence Electrons: The Key to Sodium’s Reactivity
Imagine you’re a detective, tasked with unraveling the mystery behind an element’s chemical behavior. One of the crucial clues you’ll need to decipher is the number of valence electrons, the electrons that dance around the outermost shell of an atom. Today, let’s unravel the secrets of sodium’s valence electrons and discover their profound impact on its chemistry.
Defining Valence Electrons
Picture an atom as a tiny universe, with a nucleus at its heart and electrons orbiting like celestial bodies. Valence electrons are the outermost electrons, the ones most distant from the nucleus. They’re like the adventurous explorers of the atomic realm, venturing out to interact with other atoms and determine an element’s chemical behavior.
Sodium’s Valence Electrons
Now, let’s turn our attention to sodium, a fascinating element that plays a vital role in our bodies and various industrial processes. Sodium’s atomic number is 11, which means it has 11 electrons. Using the periodic table as our guide, we determine that sodium’s electron configuration is 2-8-1. This tells us that it has two electrons in its first shell, eight in its second shell, and just one in its third and outermost shell.
Chemical Implications
That single valence electron is the key to understanding sodium’s remarkable reactivity. Valence electrons are like currency in the atomic world, and sodium’s lone valence electron makes it eager to trade. It’s willing to give up this electron to achieve a more stable electron configuration, making it a potent reducing agent. This tendency to give up electrons also explains why sodium is so highly reactive, readily forming bonds with other elements.
Understanding the number and significance of valence electrons has helped us unlock the secrets of sodium’s chemistry. Sodium’s lone valence electron makes it an active participant in chemical reactions, explaining its reactivity and reducing properties. Remember, the number of valence electrons is a fundamental characteristic of an element, and it’s essential for comprehending its chemical behavior. As we delve deeper into the world of chemistry, valence electrons will continue to guide us in unraveling the mysteries of the atomic realm.
Chemical Implications of Sodium’s Valence Electrons
Sodium, a captivating element that dances under the spotlight of chemistry, has a profound story to tell about its lone valence electron. This single electron holds the key to understanding sodium’s remarkable reactivity and its role as a crucial reducing agent.
Sodium’s atomic number, 11, reveals its electron configuration: 1s22s22p63s1. The solitary electron residing in the outermost 3s orbital is the solitary player responsible for sodium’s fascinating chemical adventures.
This lone ranger electron is highly eager to break free from sodium’s embrace, embarking on a quest for stability. This instability renders sodium an exceptional reducing agent, readily donating its valence electron to other atoms or molecules in a chemical reaction. This electron-giving nature makes sodium highly reactive, prone to forming chemical bonds with a wide range of elements.
In essence, sodium’s lone valence electron is the driving force behind its fiery reactivity. This chemical chameleon can transform itself into various compounds, often playing a crucial role in industrial processes, medical applications, and everyday life. Understanding the dance of this solitary electron is a gateway to unraveling the captivating chemistry of sodium.