Chemical Reaction Products: Understanding Types, Applications, And Predictive Power
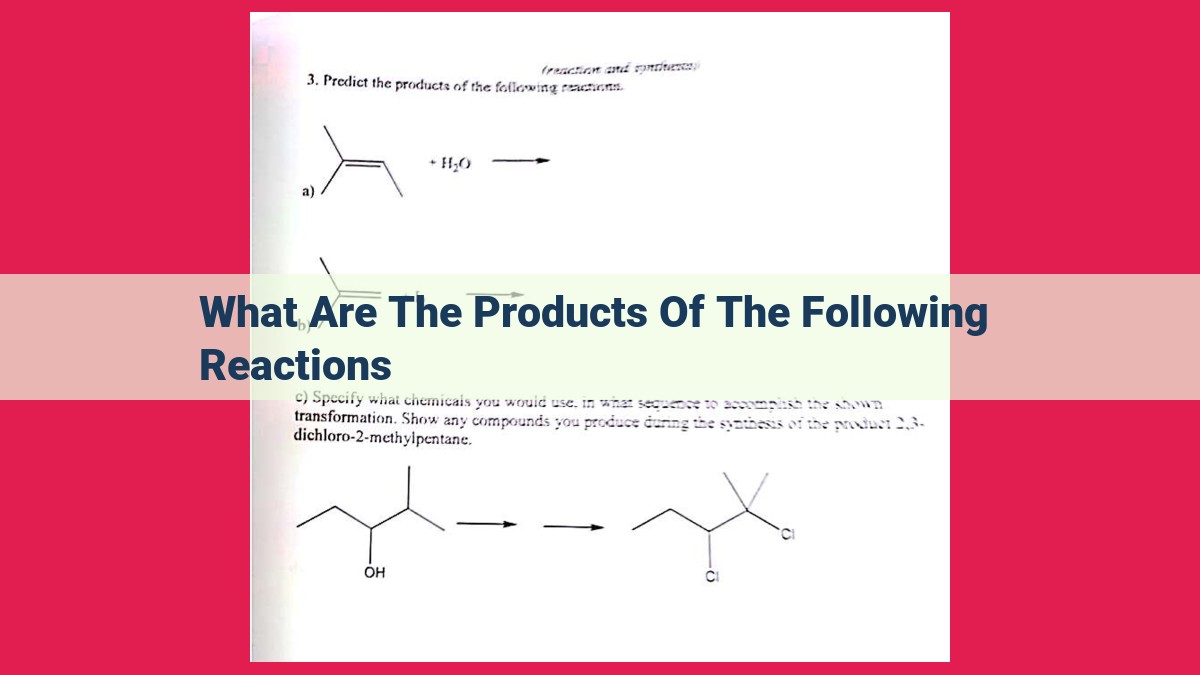
Chemical reactions produce various products, depending on the type of reaction. Addition reactions produce alkanes, alkenes, or alkynes. Elimination reactions yield alkenes, alkynes, water, or hydrogen halide. Substitution reactions result in alkyl halides, alcohols, ethers, or esters. Combustion reactions generate carbon dioxide, water, and heat. Redox reactions lead to oxidized and reduced species, as well as electrons. Understanding these products is crucial for predicting the outcome of chemical reactions and their applications in various fields.
- Define chemical reactions and their importance.
- Explain the concept of reactants and products.
The Enchanting World of Chemical Reactions
Step into the fascinating realm of chemical reactions, where elements dance and transform before your very eyes. These reactions play a crucial role in our world, shaping the materials we use, the food we eat, and even the very air we breathe.
At the heart of chemical reactions lies the concept of reactants and products. Reactants are like the ingredients in a recipe, while products are the delicious outcome. During a reaction, reactants combine to form new substances with unique properties.
Imagine a chemical reaction as a grand gathering of atoms and molecules. As they come into contact, they exchange electrons, like tiny dancers swapping partners. These electron swaps lead to the formation of new chemical bonds, creating products that are distinct from the original reactants.
Embark on a captivating journey through the diverse world of chemical reactions and discover the secrets behind the transformations that shape our world.
Products of Addition Reactions: Unraveling the Essence of Chemical Bonding
In the realm of chemistry, chemical reactions hold a pivotal role, enabling the transformation of substances into new entities. Among these reactions, addition reactions stand out as processes that involve the joining of two or more molecules or ions to form a larger molecule.
Understanding Addition Reactions: A Mechanism Unveiled
Addition reactions, often depicted by the symbol “+,” are characterized by the addition of a group or atom to an unsaturated compound. Unsaturated compounds, such as alkenes and alkynes, contain double or triple bonds between carbon atoms, respectively. These double or triple bonds serve as unsaturated sites, providing a point of entry for the addition of other atoms or groups.
The mechanism of addition reactions typically involves the breaking and formation of covalent bonds. The unsaturated compound reacts with a reactant (a substance that undergoes a chemical reaction), which is usually a polar molecule or ion. The reactant’s polar nature allows for the heterolytic bond cleavage, a process where one molecule splits into two oppositely charged ions. The ions then react with the unsaturated compound, forming new covalent bonds between the carbon atoms and the added group or atom.
Unveiling the Products of Addition Reactions
Depending on the specific reactants and conditions involved, addition reactions can yield a variety of products. Predominantly, these products belong to the following categories:
-
Alkanes: These are saturated hydrocarbons, meaning they consist of only single bonds between carbon atoms. Alkanes are formed through the addition of hydrogen atoms to an unsaturated compound, resulting in the saturation of all double or triple bonds.
-
Alkenes: These are unsaturated hydrocarbons that contain at least one double bond between carbon atoms. Alkanes are formed through the addition of a hydrogen atom to each carbon atom in a double bond, resulting in the formation of two single bonds.
-
Alkynes: These are unsaturated hydrocarbons that contain at least one triple bond between carbon atoms. Alkynes are formed through the addition of two hydrogen atoms to each carbon atom in a triple bond, resulting in the formation of two single bonds and one double bond.
Addition reactions play a crucial role in various chemical processes, from the synthesis of organic molecules to the combustion of fuels. Their ability to add atoms or groups to unsaturated compounds makes them invaluable tools in the construction of complex and diverse chemical structures. By unraveling the mechanisms and products of addition reactions, we deepen our understanding of the intricate tapestry of chemical bonding and reactions.
Products of Elimination Reactions
- Define elimination reactions and their mechanism.
- List and explain the products of elimination reactions, including alkenes, alkynes, water, and hydrogen halide.
Products of Elimination Reactions
In the realm of chemical wizardry, where atoms dance and molecules transform, elimination reactions stand out as masters of subtraction. These reactions involve the removal of atoms or groups of atoms from a substrate molecule, giving rise to a new product with a double bond or a triple bond.
Elimination reactions proceed through a concerted mechanism, where the breaking and forming of bonds occur simultaneously. This means that the transition state resembles a dance, with atoms moving in perfect synchrony to achieve the final product.
The products of elimination reactions vary depending on the nature of the substrate and the reaction conditions. Here are the key products you need to know:
-
Alkenes: These are hydrocarbons containing a double bond between two carbon atoms. Alkenes are formed when a hydrogen atom and a leaving group (such as a halogen or a hydroxyl group) are removed from adjacent carbon atoms.
-
Alkynes: Similar to alkenes, alkynes contain a triple bond between two carbon atoms. They are formed when two hydrogen atoms are removed from adjacent carbon atoms.
-
Water: Elimination reactions involving alcohols can produce water as a byproduct. This occurs when a hydrogen atom and a hydroxyl group are removed from adjacent carbon atoms.
-
Hydrogen halide: Elimination reactions involving alkyl halides can produce hydrogen halide (such as hydrogen chloride or hydrogen bromide) as a byproduct. This occurs when a hydrogen atom and a halide ion are removed from adjacent carbon atoms.
Elimination reactions are not just theoretical concepts; they find numerous applications in organic synthesis. For instance, they are used to prepare alkenes, which serve as building blocks for a wide range of polymers and other chemicals. They are also employed in the production of biofuels and pharmaceuticals.
So, next time you encounter an elimination reaction, remember the products it can generate and the dance-like mechanism that governs its outcome. These reactions are not merely about subtracting atoms; they are about creating new possibilities and shaping the molecular landscape of our world.
Products of Substitution Reactions
Embark on a Chemical Adventure: Unraveling the Secrets of Substitution Reactions
In the realm of chemistry, reactions play a pivotal role in shaping substances and driving transformations. Among the diverse types of reactions, substitution reactions stand out as fascinating processes that involve the replacement of one atom or group of atoms with another.
Delving into the Mechanism of Substitution Reactions
Substitution reactions occur via two primary mechanisms: nucleophilic substitution and electrophilic substitution. In nucleophilic substitution, a nucleophile (an electron-rich species) attacks an electrophile (an electron-deficient species), resulting in the substitution of a leaving group with the nucleophile. Conversely, electrophilic substitution involves the attack of an electrophile on a nucleophile, leading to the substitution of a hydrogen atom with the electrophile.
The Diverse Products of Substitution Reactions
The products of substitution reactions vary widely depending on the specific reactants and conditions employed. Here are some common products:
1. Alkyl Halides: Alkyl halides are organic compounds that contain a halogen atom (fluorine, chlorine, bromine, or iodine) bonded to an alkyl group (an organic group with a carbon chain). In substitution reactions, alkyl halides can be formed when a halide ion replaces a leaving group.
2. Alcohols: Alcohols are organic compounds that contain a hydroxyl group (-OH) bonded to an alkyl group. In substitution reactions, alcohols can be produced when a hydroxide ion replaces a leaving group.
3. Ethers: Ethers are organic compounds that contain an oxygen atom bonded to two alkyl groups. In substitution reactions, ethers can be formed when an alkoxide ion (RO-) replaces a leaving group.
4. Esters: Esters are organic compounds that contain a carbonyl group (C=O) bonded to an alkyl group and an alkoxy group (RO-). In substitution reactions, esters can be formed when an ester group replaces a leaving group.
The Significance of Substitution Reactions
Substitution reactions find widespread applications in organic chemistry. They are used in:
- Pharmaceutical synthesis: To prepare active ingredients for medications.
- Polymer production: To create plastics, synthetic fibers, and other materials.
- Fuel processing: To refine crude oil and produce gasoline and other fuels.
By understanding the mechanism and products of substitution reactions, chemists can manipulate organic molecules and harness their unique properties for various technological advancements.
Delving into Combustion Reactions: Understanding Their Products and Significance
Combustion reactions play a pivotal role in our daily lives, shaping processes from cooking to fueling energy sources. These chemical reactions involve the rapid combination of a substance with oxygen, releasing energy in the form of heat and light. The products of combustion reactions are not only fundamental to life but also influence our environment and technological advancements.
At the heart of combustion reactions is the union of any substance, often organic materials like wood or gasoline, with oxygen molecules. This union triggers a chain reaction, where the breaking and forming of chemical bonds releases enormous amounts of energy.
The primary products of combustion reactions are three key substances:
-
Carbon dioxide (CO2): A colorless, odorless gas that is a natural byproduct of cellular respiration and forms a major component of the Earth’s atmosphere.
-
Water (H2O): An essential compound for life, water molecules are produced when hydrogen atoms from the fuel combine with oxygen.
-
Heat: The energy released during combustion reactions manifests as heat, which can be harnessed for various purposes.
In addition to these primary products, combustion reactions can also produce other substances. For instance:
-
Nitrogen oxides (NOx): These are harmful pollutants that contribute to smog formation.
-
Sulfur oxides (SOx): These acidic compounds can cause respiratory problems and environmental damage.
-
Particulate matter: Tiny particles of soot, ash, and other substances that can have negative health effects and contribute to air pollution.
The significance of combustion reactions extends beyond their role in energy production. These reactions play a crucial part in natural processes, such as forest fires and the cycling of carbon through the environment. Combustion reactions also form the basis of many industrial processes, from metallurgy to waste disposal.
Understanding the products and importance of combustion reactions not only enhances our appreciation for the natural world but also empowers us to harness their potential while mitigating their environmental impact.
Products of Redox Reactions
- Define redox reactions and their mechanism.
- Explain the concepts of oxidation and reduction.
- Describe the products of redox reactions, including oxidized and reduced species, and electrons.
Redox Reactions: Unraveling the Story of Electron Exchange
In the realm of chemistry, redox reactions hold a captivating place. They are like the dance between atoms, where electrons gracefully move from one partner to another, leading to a kaleidoscope of chemical transformations. Let’s delve into the captivating world of redox reactions and unravel their enchanting tale.
The Essence of Redox: A Tale of Two Parts
Redox reactions are defined by their two intertwined processes: oxidation and reduction. Oxidation is when a substance loses electrons, while reduction occurs when it gains electrons. Imagine a shy electron bidding farewell to its current home and setting off on a new adventure with a more inviting atom.
The Products of Redox: A Symphony of Transformations
The outcome of a redox reaction is a symphony of chemical changes. The products of these reactions can be diverse, ranging from oxidized and reduced species to electrons dancing freely. Oxidized species have lost electrons, while reduced species have gained them. Like the yin and yang of chemistry, these species represent the harmonious balance of electron transfer.
Electrons: The Unseen Forces Shaping Change
In redox reactions, electrons play a pivotal role. They are the invisible threads that weave together the tapestry of chemical transformations. As electrons shift from one atom to another, they create a cascade of changes, like ripples in a tranquil pond. Electrons are the unsung heroes of redox reactions, their dance invisible yet essential.
Examples that Bring the Story to Life
Let’s ignite our curiosity with a few real-life examples of redox reactions. When iron rusts, we witness the oxidation of iron and the reduction of oxygen. The rust that forms is the oxidized iron compound, while the oxygen gains electrons in the process. Another captivating example is the combustion of fuels, where the fuel undergoes oxidation, and the oxygen is reduced. The warmth and light we experience from burning fuels are testaments to the power of redox reactions.
Redox Reactions: A Window into the Dynamic World of Chemistry
Redox reactions are not merely abstract concepts but living, breathing processes that shape our world. They are at the heart of countless chemical transformations, from the rusting of iron to the combustion of fuels. By embracing the storytelling essence of redox reactions, we gain a deeper understanding of the intricate dance of electrons, oxidation, and reduction, unraveling the captivating saga of chemical change.