Mastering Chemical Nomenclature: A Guide To Iupac Rules
The IUPAC name, governed by the International Union of Pure and Applied Chemistry (IUPAC), is a systematic and globally recognized nomenclature system for uniquely identifying chemical compounds. It provides a precise and consistent way to describe a molecule’s structure, atomic composition, functional groups, and molecular geometry. By following IUPAC’s specific rules, chemists can assign a unique IUPAC name to each compound, enabling accurate identification, unambiguous communication, and comprehensive understanding of chemical structures.
Understanding IUPAC Nomenclature
- Definition of IUPAC and its role in chemistry
- Importance of systematic naming for accurate identification and description of chemical structures
Understanding IUPAC Nomenclature: The Key to Accurate Chemical Identification
In the vast realm of science, the language of chemistry plays a crucial role in unraveling the intricate secrets of the molecular world. IUPAC, an acronym for the International Union of Pure and Applied Chemistry, serves as the global authority in defining the rules and standards for chemical nomenclature.
The Importance of IUPAC Nomenclature
Imagine a world without systematic naming conventions in chemistry. Chaos would reign as scientists struggled to accurately identify and describe the countless compounds that shape our universe. IUPAC nomenclature serves as a beacon of clarity, providing a common language for scientists around the world to communicate about chemical structures and properties with precision.
By assigning unique names to chemical compounds, IUPAC ensures that every substance has a distinctive identity. This systematic approach facilitates the accurate identification, classification, and understanding of these compounds. It enables chemists to share research findings, collaborate in drug discovery, and develop new materials with confidence.
The Anatomy of an IUPAC Name
Each IUPAC name is a carefully crafted expression that conveys a wealth of information about a chemical structure. It typically consists of three parts:
- Prefix: Indicates the number of carbon atoms in the parent chain.
- Infix: Describes any double or triple bonds present in the chain.
- Suffix: Reveals the functional group that characterizes the compound.
For example, the IUPAC name “1-butene” tells us that the compound has a chain of four carbon atoms (but-), one double bond (-en), and a terminal carbon-carbon double bond (1-).
Distinct Names for Isomers
Isomers are compounds that share the same molecular formula but differ in their atomic arrangement. IUPAC nomenclature distinguishes between isomers by assigning them unique names that reflect their structural differences. This distinction is crucial for understanding the properties and reactivity of these compounds.
For instance, butane and isobutane are isomers with the same molecular formula (C4H10). However, butane has a straight-chain structure, while isobutane has a branched structure. IUPAC nomenclature reflects this difference by naming butane as 1-butane and isobutane as 2-methylpropane.
Concept of the IUPAC Name
The International Union of Pure and Applied Chemistry (IUPAC) has standardized the naming of chemical compounds to ensure clear and uniform communication among scientists and researchers worldwide. IUPAC names serve as unique identifiers for each chemical substance, providing valuable information about its structure, composition, and properties.
Each IUPAC name is meticulously crafted to convey specific details about the molecule it represents. By carefully dissecting the name, chemists can discern the compound’s carbon skeleton, functional groups, and even its molecular geometry. This comprehensive information empowers scientists to accurately identify, describe, and classify chemical substances, facilitating effective communication and the advancement of chemistry.
Handling Isomers in IUPAC Naming: Unraveling the Mystery of Molecular Equivalence
When delving into the intriguing world of chemistry, we encounter molecules – the building blocks of all matter. These molecules can arrange themselves in various ways, giving rise to intriguing counterparts known as isomers. Isomers, like shape-shifting puzzles, share the same molecular formula but possess distinct atomic arrangements, akin to pieces of a jigsaw that fit together differently.
The International Union of Pure and Applied Chemistry (IUPAC), the governing body of chemical nomenclature, has devised a systematic naming system that plays a crucial role in identifying and differentiating these isomeric doppelgangers. IUPAC names serve as unique identifiers for each molecule, providing a comprehensive description of its structure, atoms, functional groups, and molecular geometry.
In the realm of isomers, IUPAC nomenclature shines as a master decipherer, distinguishing between molecules that may appear identical but behave in distinct ways. By dissecting the atomic arrangement of each isomer, IUPAC names reveal subtle differences that would otherwise remain hidden.
For instance, consider the pair of isomers, C4H10. Both molecules are composed of the same number of carbon and hydrogen atoms, but their structures differ vastly. One isomer, butane, arranges its carbon atoms in a straight chain, while the other, 2-methylpropane, adopts a branched structure. IUPAC names capture these structural variations, ensuring that each isomer has a unique label that accurately reflects its molecular blueprint.
Isomers, like enigmatic twins, often exhibit contrasting properties, influencing their reactivity and behavior in chemical reactions. By providing precise molecular descriptions, IUPAC names empower chemists to predict and understand the distinct characteristics of isomers, enabling them to unravel the complexities of chemical reactions and design tailored experiments.
Embracing the systematic approach of IUPAC nomenclature, we gain the ability to decode the cryptic language of molecules, unlocking a deeper understanding of their chemistry and behavior. Just as a key opens a lock, IUPAC names unlock the secrets of molecular structure, empowering us to navigate the vast and fascinating world of chemistry with confidence.
Chemical Nomenclature: Principles and Conventions
- Introduction to chemical nomenclature and its purpose
- IUPAC’s specific rules for describing molecular structure
- Conventions for prefixes, suffixes, and prefixes for substituents
- Importance of consistency and unambiguity in IUPAC naming
Chemical Nomenclature: Orchestrating Order in the Chemical Cosmos
Imagine a bustling metropolis, where every street, avenue, and alleyway has a unique address, guiding people to their destinations with precision. Similarly, in the realm of chemistry, each molecule requires a systematic and unambiguous naming system to facilitate precise communication and accurate identification. Enter IUPAC nomenclature, the universal language of chemistry.
Principles and Conventions of IUPAC Nomenclature
Developed by the International Union of Pure and Applied Chemistry (IUPAC), these principles establish a standardized set of rules for describing molecular structures. By following these guidelines, chemists worldwide can confidently assign unique names to even the most complex compounds.
Dissecting the Name: A Blueprint of Structure
IUPAC names are intricate yet informative, providing a wealth of details about a molecule’s structure. The name begins with the root, indicating the carbon chain length. Prefixes, such as “meth-“, “eth-“, and “prop-“, denote the number of carbons. Suffixes, like “-ane”, “-ene”, and “-yne”, reveal the type of bonding between the carbons.
Substituents, atoms or groups attached to the main chain, are named using prefixes like “methyl-“, “ethyl-“, and “isopropyl-“. Their position along the chain is indicated by a number. For example, “2-methylbutane” indicates a butane chain with a methyl group attached to the second carbon.
Consistency and Unambiguity: The Cornerstones of IUPAC Nomenclature
Consistency is crucial in IUPAC naming. Each molecule should have only one correct IUPAC name. This ensures that scientists can refer to compounds unambiguously, avoiding confusion and miscommunication.
Moreover, IUPAC nomenclature strives for maximum clarity. The name should concisely convey the molecular structure, including the number of atoms, functional groups, and any isomeric forms. This enables chemists to visualize and understand the molecule’s properties and reactivity.
By adhering to the principles and conventions of IUPAC nomenclature, chemists create a structured language that allows them to navigate the vast landscape of chemical compounds with precision and efficiency.
Unlocking the Secrets of IUPAC Nomenclature: A Comprehensive Guide
In the realm of chemistry, precise communication is paramount. Just as words form the building blocks of language, IUPAC nomenclature serves as the universal language for naming chemical compounds. Understanding this systematic approach empowers scientists and researchers to accurately identify and describe the intricate structures of molecules.
The Concept of IUPAC Names
Each IUPAC name is a unique identifier, providing a comprehensive description of a molecule’s structure, atoms, functional groups, and molecular geometry. These names empower chemists to convey complex chemical information concisely and unequivocally.
Navigating Isomers with IUPAC Naming
Isomers, compounds with the same molecular formula but different atomic arrangements, pose a challenge in nomenclature. However, IUPAC names effectively distinguish between isomers based on their precise atomic configurations. By unraveling the subtleties of isomeric structures, IUPAC nomenclature ensures clarity in chemical communication.
Principles and Conventions of Chemical Nomenclature
IUPAC’s rigorous guidelines govern the naming of molecules. These rules dictate the use of specific prefixes, suffixes, and substituent terms. Adhering to these conventions guarantees consistency and unambiguity in naming, fostering seamless communication among chemists worldwide.
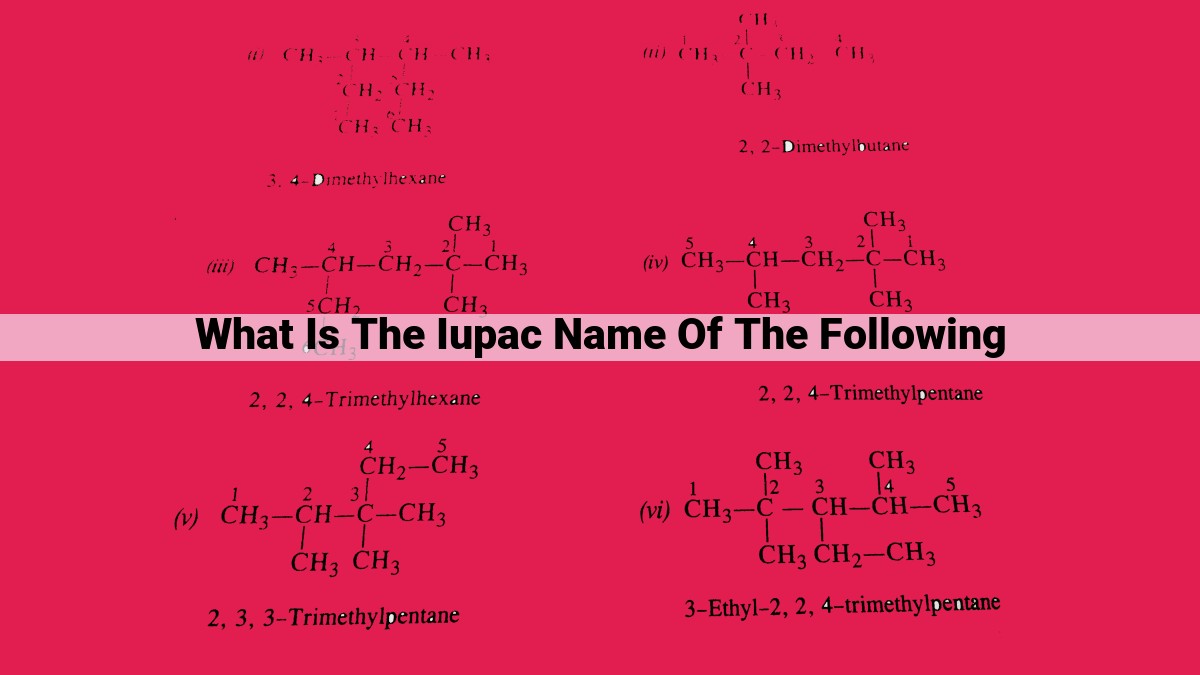
Determining the IUPAC Name of a Molecule
To illustrate the practical application of IUPAC nomenclature, let’s embark on a step-by-step journey to derive the name of a sample molecule:
Example: Unraveling the IUPAC Name of 2-Methylbut-1-ene
-
Step 1: Carbon Count and Base Name
-
This molecule has a 4-carbon chain, which corresponds to the base name “but-“.
-
Step 2: Functional Group Identification
-
The presence of a double bond indicates an alkene functional group, denoted by the suffix “-ene”.
-
Step 3: Substituent Analysis
-
A methyl group (-CH3) is attached to the second carbon, reflected by the prefix “2-methyl”.
-
Step 4: Combining the Elements
-
Combining the base name, suffix, and prefix yields the complete IUPAC name: 2-methylbut-1-ene.
By following these principles, chemists can confidently navigate the intricacies of IUPAC nomenclature, unlocking a world of accurate and precise chemical communication.