Master Chemical Nomenclature With Iupac Guidelines: Unlocking Precise Communication
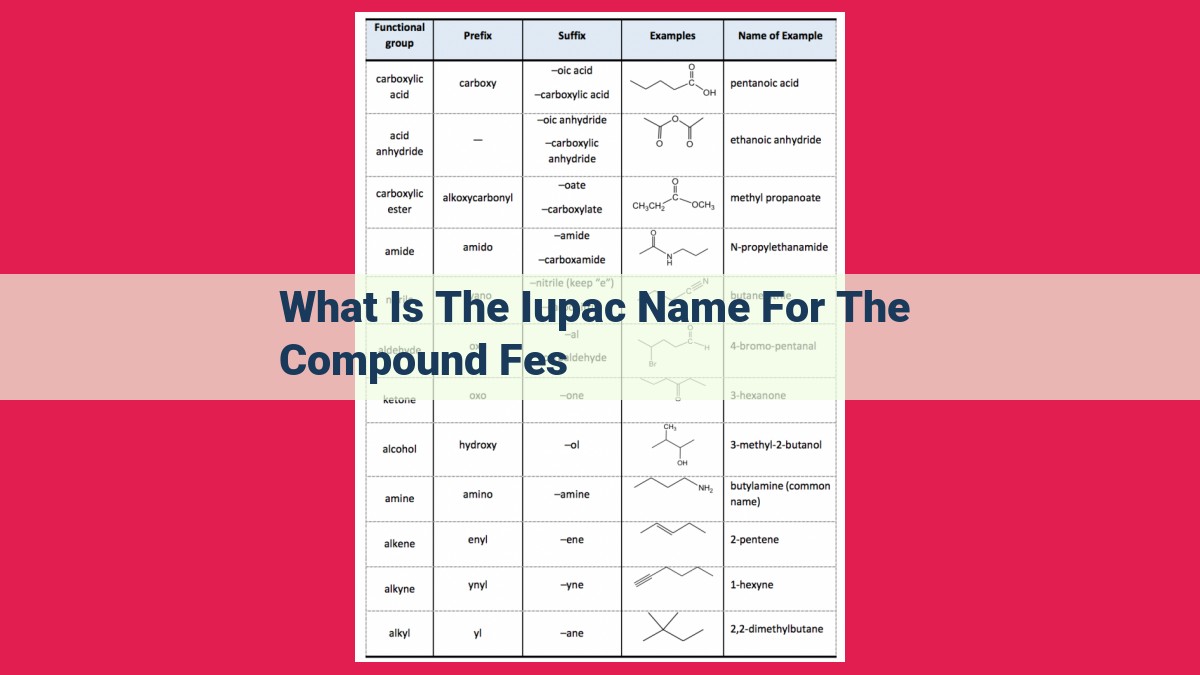
Standardized nomenclature in chemistry is crucial for precise communication. IUPAC guidelines ensure a systematic approach to naming compounds. The IUPAC name for FeS is iron(II) sulfide. This name reflects the oxidation state of iron (+2) and the presence of the sulfide anion (S2-). IUPAC nomenclature is essential in both organic and inorganic chemistry, aiding in the identification and understanding of chemical species.
Understanding the Language of Chemistry: The Importance of Standardized Nomenclature
In the intricate world of chemistry, where countless chemical compounds exist, the ability to clearly and accurately identify these substances is paramount. Standardized nomenclature, the systematic naming of compounds, plays a pivotal role in ensuring effective scientific communication and avoidance of confusion.
One of the most influential organizations in establishing guidelines for chemical nomenclature is the International Union of Pure and Applied Chemistry (IUPAC). Since 1919, IUPAC has dedicated itself to developing and disseminating standardized rules for naming chemical compounds, ensuring a universal language for chemists worldwide.
Understanding IUPAC Nomenclature: A Systematic Approach to Naming Compounds
The Language of Chemistry: IUPAC Nomenclature
In the vast realm of chemistry, a common language is essential for clear communication and precise identification of chemical substances. The International Union of Pure and Applied Chemistry (IUPAC) has established guidelines known as IUPAC nomenclature to provide a standardized system for naming compounds. This standardized approach ensures that scientists worldwide can understand each other and accurately represent the structures of chemical species.
Basic Principles and Conventions
IUPAC nomenclature relies on a set of fundamental principles and conventions to assign unique and systematic names to compounds. One key principle is molecular structure, which forms the basis for naming. The root name of a compound indicates the number of carbon atoms in its parent hydrocarbon chain. For example, “ethane” denotes a two-carbon chain, “propane” denotes a three-carbon chain, and so on.
Another important convention is the use of prefixes, which indicate the number and position of different substituents (or attached functional groups) on the parent chain. Prefixes such as “methyl-“, “ethyl-“, and “propyl-” designate the specific carbon groups attached. For instance, in the compound “2-methylpropane,” the “methyl-” prefix indicates that a methyl group is attached to the second carbon atom of the propane chain.
Logical and Systematic Approach
IUPAC nomenclature provides a highly logical and systematic approach to naming compounds. It ensures that compounds with similar structures are given similar names, while compounds with different structures have distinct names. This systematic approach allows chemists to predict the IUPAC name of a compound based on its structure and vice versa.
It also facilitates the retrieval of chemical information. By searching for a name in a database, scientists can access a wealth of information about the compound’s properties, structure, and reactivity. IUPAC nomenclature serves as the common denominator that connects chemical databases worldwide.
Benefits of IUPAC Nomenclature
The benefits of IUPAC nomenclature are multifaceted. First, it promotes scientific accuracy and precision in chemical communication. Scientists can confidently rely on IUPAC names to accurately identify and describe chemical substances. Second, it enhances international collaboration by providing a universal language for chemists across the globe. Third, it streamlines the process of chemical data storage and retrieval, enabling scientists to access and exchange information efficiently.
Understanding the IUPAC Name for FeS: Unveiling the Symphony of Chemistry
The world of chemistry is a realm of intricate structures and complex interactions, where precise communication is paramount. Amidst this symphony of elements and compounds, the International Union of Pure and Applied Chemistry (IUPAC) emerges as the master conductor, orchestrating a universal language for the naming of chemical species.
At the heart of this language lies IUPAC nomenclature, a set of guidelines that ensure clarity and consistency in chemical communication. By following these rules, chemists can accurately describe even the most complex compounds, enabling seamless exchange of scientific knowledge and understanding.
One such compound that exemplifies the power of IUPAC nomenclature is FeS. This simple yet intriguing molecule is composed of iron (Fe) and sulfur (S) atoms. To assign its IUPAC name, we embark on a journey of chemical analysis and precision.
Prefixing the Molecular Symphony
The first step in unraveling the IUPAC name of FeS is to determine the oxidation states of its elemental constituents. As a transition metal, iron can exhibit multiple oxidation states, but in this compound, it exists in the +2 oxidation state. Sulfur, on the other hand, takes on a -2 oxidation state.
Unveiling the Anionic Harmony
In FeS, sulfur exists as an anion, which means it has gained two electrons and carries a negative charge. The suffix “-ide” is appended to the root name of the anion to indicate its charged state, resulting in the name sulfide.
Assembling the IUPAC Symphony
With the oxidation states and anionic identity established, we can now assemble the complete IUPAC name for FeS. By combining the root names of the elements and their respective oxidation states, we arrive at the elegant and precise designation:
Iron(II) sulfide
Decoding the Nomenclature
This name meticulously conveys the following information:
- Iron(II): Iron is present in the +2 oxidation state.
- Sulfide: Sulfur exists as an anion with a -2 charge.
Significance of IUPAC Nomenclature
The significance of IUPAC nomenclature extends far beyond FeS. It provides a universal language for chemists to accurately describe and identify a vast array of chemical species. This standardized approach facilitates scientific communication, enables collaboration, and ensures that chemical information is accessible and comprehensible to all.
Moreover, IUPAC nomenclature plays a crucial role in safety, ensuring that chemical hazards are properly identified and communicated. By providing clear and unambiguous names, it helps to prevent misidentification and accidental reactions.
IUPAC nomenclature is a testament to the power of standardized language in the advancement of scientific knowledge. By establishing a comprehensive and logical system for naming chemical compounds, it empowers chemists to communicate with clarity and precision, unlocking the mysteries of the molecular world.
Related Concepts: A Deeper Dive into the World of Nomenclature
Importance in Organic and Inorganic Chemistry
IUPAC nomenclature serves as the cornerstone for establishing uniformity and clarity in both organic and inorganic chemistry. It provides a consistent framework for naming even the most complex compounds, ensuring that scientists worldwide can communicate about chemical structures unambiguously.
In organic chemistry, IUPAC nomenclature enables the systematic naming of hydrocarbons, functional groups, and macromolecules. It plays a crucial role in identifying and classifying these compounds, facilitating effective research and communication.
In inorganic chemistry, IUPAC nomenclature provides a logical approach to naming inorganic compounds, including simple binary compounds, coordination complexes, and polyatomic ions. This standardization allows chemists to precisely describe the structure and composition of these compounds, enabling clear communication and understanding of their properties and reactions.
Key Concepts Related to Iron and Sulfur
The nomenclature of iron (Fe) and sulfur (S) compounds is based on their oxidation states and the type of bonding between them. In the case of FeS, the compound exists as an ionic solid where the iron is present in the +2 oxidation state (Fe²⁺) and sulfur is present as the sulfide ion (S²⁻).
This specific oxidation state of iron is indicated by the use of the Roman numeral “II” in the name, FeS. The presence of the sulfide ion is indicated by the suffix “-ide,” signifying the negative charge on the ion. The combination of these two factors results in the systematic and accurate naming of the compound as iron(II) sulfide.