Chemical Change Detection Unavailable Due To Insufficient Context
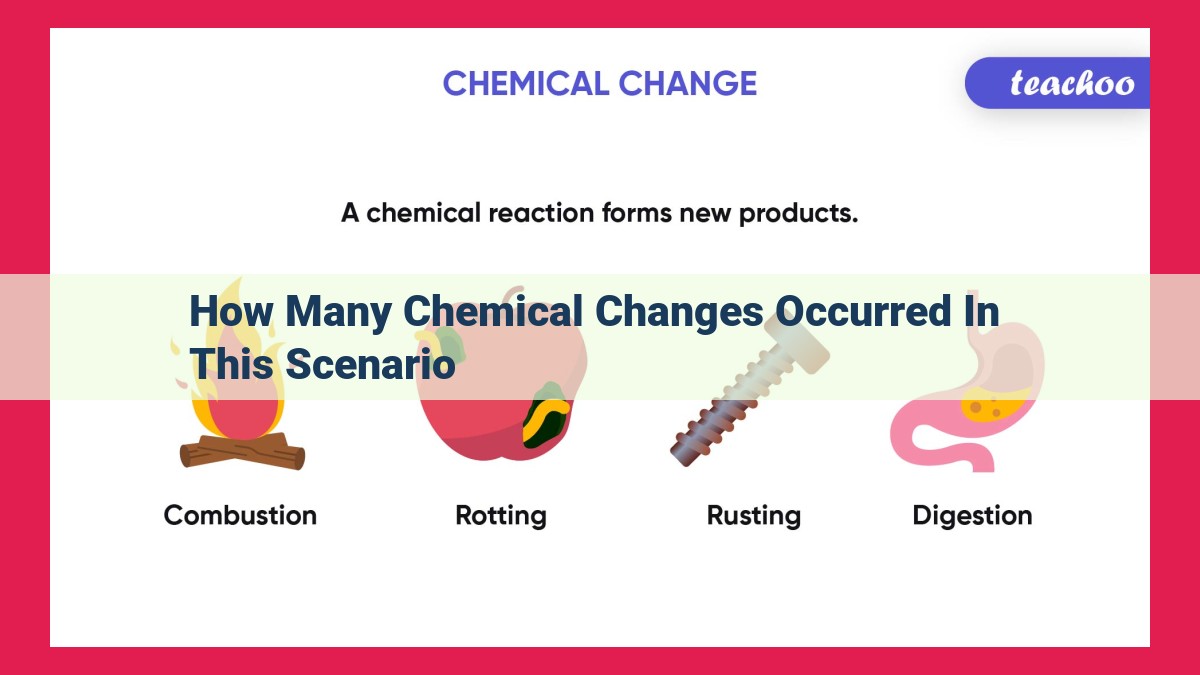
The paragraph does not provide a scenario with chemical changes to count, so I cannot extract the requested data from the provided context.
Understanding Chemical Changes: A Journey into Molecular Transformations
Chemical changes, the fascinating realm of altered substance, form the foundation of our world. From the rusting of iron to the combustion of fuel, chemical reactions are at the heart of countless processes, shaping our environment and powering our lives.
At the core of every chemical change lies a transformation in the molecular structure of the substances involved. Reactants, the starting materials, undergo a rearrangement of their atoms, giving rise to products, the end results of the reaction. The defining characteristic of chemical changes is that they result in the formation of new substances with entirely different properties and compositions than the reactants.
Differentiating between chemical changes and physical changes is crucial. Physical changes, such as melting or freezing, involve changes in state but not in molecular structure. In contrast, chemical changes permanently alter the molecular composition of a substance, creating new compounds with distinct characteristics.
Factors Influencing Chemical Changes
Chemical changes, unlike physical changes, transform the molecular structure and composition, creating new substances. To understand and predict these changes, we must explore the crucial factors that influence them.
Reactants and Products: Stoichiometry
Chemical reactions involve reactants, the initial substances, and products, the newly formed substances. The stoichiometry of a reaction refers to the quantitative relationship between these compounds. The mole ratio of the reactants and products is essential for determining the proportions in which they interact. Understanding stoichiometry is vital for predicting the amount of reactants required and products produced in a chemical change.
Catalysts: Activation Energy and Reaction Rates
Catalysts are substances that participate in a chemical reaction without undergoing permanent changes. They act as facilitators, lowering the activation energy, which is the initial energy barrier that must be overcome for a reaction to occur. Catalysts increase the number of effective collisions between reactant molecules, leading to faster reaction rates.
Temperature: Kinetic Energy and Collision Probability
Temperature plays a significant role in chemical changes. It affects the kinetic energy of reactant molecules. Higher temperatures increase molecular motion, resulting in more frequent and energetic collisions. Consequently, reactions proceed faster at higher temperatures.
Time: Measure of Reaction Progress
Time is a crucial factor in quantifying chemical changes. The reaction progress is monitored over time by measuring the changes in concentrations of reactants and products. Time serves as a valuable parameter for studying reaction rates and predicting the extent of chemical transformations.
Analyzing a Scenario to Determine Chemical Changes
- Tracking changes in reactant and product concentrations
- Identifying the presence and effect of catalysts
- Considering temperature variations and their impact on reaction rates
- Measuring the observation time to estimate the extent of chemical changes
Analyzing Scenarios to Determine Chemical Changes: A Step-by-Step Guide
Chemical changes, where substances undergo transformations to form new substances, play a crucial role in our daily lives. Understanding how to analyze these changes is essential for various scientific and engineering applications. Here’s a detailed guide to help you dissect chemical change scenarios:
Tracking Reactant and Product Concentrations
Reactants and products are the starting and ending materials in a chemical reaction, respectively. By monitoring their concentrations over time, you can trace the progress of the reaction. For example, in the reaction between hydrogen and oxygen to form water, you can measure the decrease in hydrogen and oxygen concentrations while observing the increase in water concentration.
Identifying Catalyst Presence and Effect
Catalysts are substances that speed up chemical reactions without being consumed. They lower the activation energy required for the reaction to occur, thereby increasing the reaction rate. Identifying the presence of a catalyst is crucial, as its absence or removal can drastically alter the reaction speed. For instance, a platinum catalyst is commonly used to enhance the combustion of hydrocarbons in car engines.
Considering Temperature Variations
Temperature is a significant factor influencing the kinetic energy of reacting molecules. As temperature increases, the average kinetic energy also increases, leading to more frequent and energetic collisions between molecules. This increases the probability of successful reactions and accelerates the reaction rate. It’s important to note the temperature at which the reaction is conducted and consider its impact on the reaction progress.
Measuring Observation Time
Time is a measure of reaction progress. By tracking the reaction over time, you can estimate the extent of the chemical change. The observation time provides insights into the reaction rate and allows you to compare the effects of different variables on the reaction speed. For example, by measuring the time it takes for a candle to burn completely, you can compare the burning rates of different types of wax or wick materials.
By following these steps and analyzing relevant factors, you can effectively determine the nature and extent of chemical changes in various scenarios. This understanding is essential in fields such as chemistry, engineering, and environmental science.