Understanding Chemical Buffers: Maintaining Optimal Ph Levels In Blood And Cells
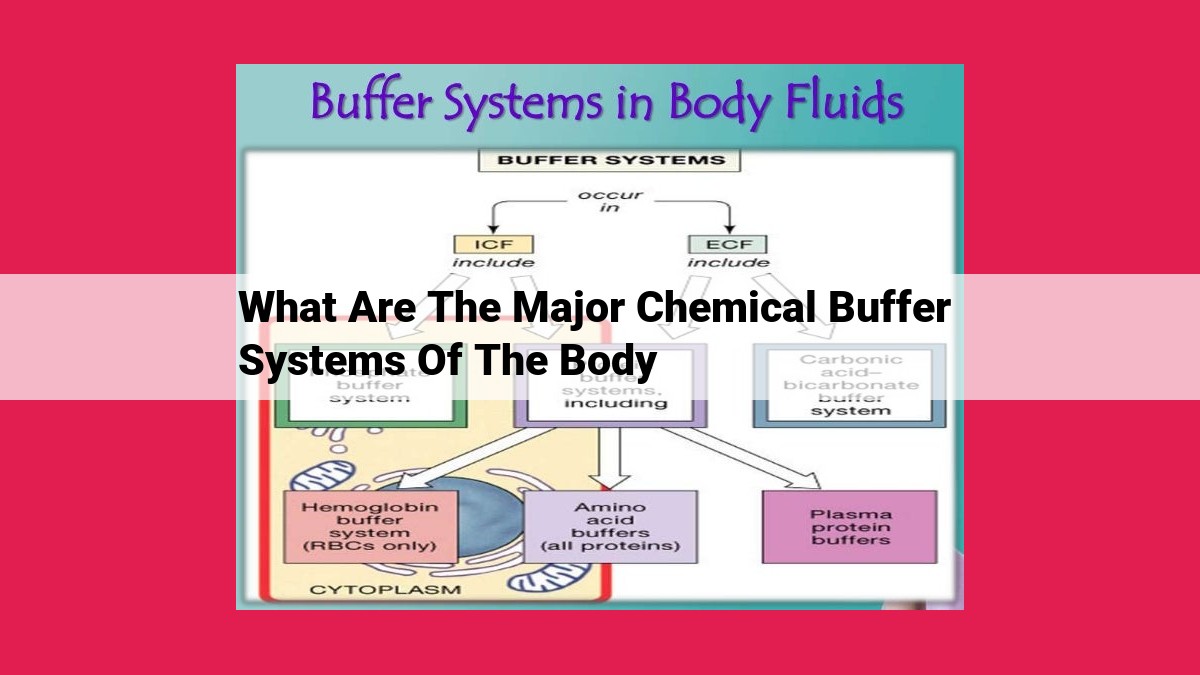
The major chemical buffer systems maintain optimal pH levels in the body: blood buffers (bicarbonate, phosphate, protein) stabilize blood pH, while intracellular buffers (bicarbonate, phosphate, protein) regulate cell pH. The bicarbonate buffer system, aided by carbonic anhydrase, plays a primary role by converting CO2 to H+ and HCO3-, maintaining acid-base balance. Phosphate and protein buffer systems also contribute to pH stability. Hemoglobin enhances both blood and intracellular buffering.
Blood Buffers
- Discuss the role of blood buffers in maintaining blood pH within a narrow range.
- Include the bicarbonate, phosphate, and protein buffer systems.
Blood Buffers: Guardians of pH Equilibrium
The human body is a finely tuned machine, and its pH levels are of paramount importance for maintaining optimal health. Just as Goldilocks sought a porridge that was neither too hot nor too cold, our blood pH must be just right to support countless biochemical reactions.
Enter blood buffers, the unsung heroes that stabilize pH within a narrow range. These molecular guardians include bicarbonate, phosphate, and protein buffer systems. Each system operates like a pH-regulating dance, ensuring that our blood pH remains steadfast amidst the constant release of acids and bases.
Bicarbonate Buffer: The Primary Player
The bicarbonate buffer system is the most significant player in this pH balancing act. It involves a dynamic interplay between carbonic acid (H2CO3) and bicarbonate ion (HCO3-). When acids enter the bloodstream, carbonic anhydrase, an enzyme present in red blood cells, jumps into action, converting the acid into H2CO3. This H2CO3 then dissociates into H+ and HCO3-. The H+ ions are neutralized by HCO3-, preventing a drastic pH shift.
Phosphate Buffer: A Supporting Role
While not as prominent as the bicarbonate buffer, the phosphate buffer system also plays a crucial role. It involves phosphate ions (HPO42-) and dihydrogen phosphate ions (H2PO4-), which buffer changes in pH by accepting or releasing H+ ions.
Protein Buffer: The Versatility of Amino Acids
Proteins, the workhorses of the body, also contribute to pH stability. Their amino acid side chains possess acidic or basic properties, allowing them to act as buffers. Hemoglobin, the oxygen-carrying molecule in red blood cells, is a notable protein buffer that helps regulate pH in both the blood and within cells.
Blood buffers are essential for maintaining pH homeostasis, allowing the body to function optimally. By continuously neutralizing acids and bases, they ensure that our blood pH remains within the narrow range necessary for life. These remarkable molecular guardians work tirelessly behind the scenes, ensuring that our bodies remain in perfect harmony.
The Vital Role of Intracellular Buffers in Maintaining Cellular Equilibrium
Within the intricate confines of our cells, a delicate dance of chemical reactions takes place, each essential for life’s processes. However, these reactions are sensitive to pH, and even slight deviations can disrupt cellular harmony. Enter intracellular buffers, the unsung heroes of this biochemical symphony.
Imagine your cells as a finely tuned orchestra, where every musician (enzyme) must play in perfect harmony. pH stability is the conductor’s baton, ensuring that the ensemble performs flawlessly. Intracellular buffers act as the backstage crew, diligently maintaining this delicate balance.
Just as blood buffers guard the pH of our bloodstream, intracellular buffers steadfastly protect the pH of our cells. The bicarbonate, phosphate, and protein buffer systems stand ready to neutralize any potential acid or base disturbances, ensuring that the cellular environment remains conducive to life.
Bicarbonate Buffer System: The maestro of intracellular buffering, the bicarbonate buffer system uses carbonic anhydrase to convert carbon dioxide (CO2) into hydrogen ions (H+) and bicarbonate ions (HCO3-). This enzymatic tango buffers against both acids and bases, ensuring pH stability.
Phosphate Buffer System: A less prominent but equally important player, the phosphate buffer system employs hydrogen phosphate ions (HPO42-) and dihydrogen phosphate ions (H2PO4-) to neutralize pH changes.
Protein Buffer System: Proteins, the workhorses of our cells, also lend their buffering abilities. Amino acids with charged side chains can donate or accept protons, helping to stabilize pH.
Hemoglobin: A Versatile Buffer within Cells
Hemoglobin, famed for its respiratory role, also moonlight as an intracellular buffer. It uses its histidine residues to bind protons, effectively preventing pH fluctuations.
Acid-Base Balance and the Henderson-Hasselbalch Equation
Acid-base balance, the harmonious coexistence of acids and bases, is carefully regulated by buffer systems. The Henderson-Hasselbalch equation quantifies this delicate balance, calculating pH based on the concentrations of weak acids and their conjugate bases.
Intracellular buffers are the silent guardians of our cells, ensuring pH stability amidst the constant biochemical flux. Their tireless efforts allow enzymes to function optimally, maintaining cellular equilibrium and the symphony of life. Understanding the intricate interplay of these buffer systems is essential for appreciating the delicate balance that sustains our very existence.
The Bicarbonate Buffer System: A Vital Player in Maintaining Acid-Base Balance
In the intricate symphony of our body’s chemistry, the bicarbonate buffer system plays a pivotal role in maintaining the delicate balance of pH, the measure of acidity or alkalinity. This system is the body’s primary buffer, working tirelessly to prevent drastic shifts in pH levels that could disrupt vital physiological processes.
At its core, the bicarbonate buffer system involves the conversion of carbon dioxide (CO2) into hydrogen ions (H+) and bicarbonate ions (HCO3-). This conversion is facilitated by the enzyme carbonic anhydrase, which acts as a catalyst, speeding up the reaction.
The H+ ions released by the buffer system can neutralize hydroxide ions (OH-), which would otherwise raise the pH of the blood. Conversely, the HCO3- ions can combine with H+ ions to form carbonic acid (H2CO3), which can then break down into CO2 and H2O. This reversible reaction allows the buffer system to both absorb and release H+ ions as needed, maintaining a steady-state pH level in the bloodstream.
The Henderson-Hasselbalch equation provides a mathematical framework for understanding the interplay between the bicarbonate buffer system and acid-base balance. This equation states that pH is directly proportional to the ratio of HCO3- to dissolved CO2 in the blood. By adjusting the concentration of either HCO3- or CO2, the body can fine-tune the pH towards the optimal range of 7.35 to 7.45.
The bicarbonate buffer system is intimately connected to the respiratory and renal systems. The lungs control CO2 levels through respiration, while the kidneys regulate HCO3- concentration through the excretion or reabsorption of bicarbonate ions. This intricate interplay ensures that the pH of the blood remains within the narrow range necessary for optimal cellular function and overall health.
The Phosphate Buffer System: A Guardian of pH Stability
Within the intricate symphony of life, pH balance reigns supreme. From the bustling metropolis of our cells to the vast expanse of our blood, maintaining a stable pH is paramount for life’s delicate dance. Enter the phosphate buffer system, a guardian of pH stability, silently working to ensure the proper functioning of our biological orchestra.
The phosphate buffer system, like a diligent custodian, meticulously stabilizes pH levels by absorbing or releasing hydrogen ions (H+). Its strength lies in the reversible acid-base reactions involving phosphoric acid (H3PO4) and its conjugate base, hydrogen phosphate (H2PO4-).
H3PO4 + H2O ⇌ H2PO4- + H+
The Henderson-Hasselbalch equation, like a mathematical wizard, quantifies this delicate balance:
pH = pKa + log([H2PO4-] / [H3PO4])
Where:
- pKa is the dissociation constant for the phosphate buffer system
- [H2PO4-] is the concentration of hydrogen phosphate
- [H3PO4] is the concentration of phosphoric acid
By manipulating the ratio of [H2PO4-] to [H3PO4], the phosphate buffer system can maintain pH within a narrow range. When excess H+ ions threaten to disrupt this balance, the system’s innate tendency is to convert H2PO4- into H3PO4, absorbing the excess H+ and restoring equilibrium. Conversely, when H+ ions are depleted, the system releases H+ ions from H3PO4 to replenish the supply.
The phosphate buffer system, though less prominent than its counterpart, the bicarbonate buffer system, plays a crucial role in intracellular pH regulation. Within the confines of our cells, it meticulously buffers H+ ions, ensuring a stable pH for optimal enzyme activity and cellular function.
The phosphate buffer system, often overlooked but eternally diligent, stands as a testament to the intricate mechanisms that sustain life. Its ability to buffer H+ ions and stabilize pH levels enables the harmonious functioning of our cells and organs. As we continue to unravel the secrets of our biological symphony, let us not forget the unsung heroes like the phosphate buffer system, silently ensuring the rhythm of life remains uninterrupted.
Protein Buffer System: The pH Protectors
Within the intricate tapestry of our bodies, a battle rages silently, a constant struggle to maintain the delicate balance of pH. In this battle against acidity and alkalinity, the protein buffer system stands as an unsung hero, a guardian of cellular harmony.
pH, a measure of the acidity or alkalinity of a solution, is crucial for life. It influences the intricate biochemistry of enzymes, the function of proteins, and even the beating of our hearts. Blood pH must be maintained within a narrow range of 7.35 to 7.45 to ensure optimal cellular functioning.
The protein buffer system, like a wise sentinel, shields our blood and cells from wide pH fluctuations. These proteins, with their side chains adorned with acidic or basic groups, act as chemical buffers, soaking up excess protons (H+) or hydroxide ions (OH-) to prevent drastic pH shifts.
They collaborate seamlessly with other buffer systems, such as the bicarbonate and phosphate systems, creating a symphony of pH control. The Henderson-Hasselbalch equation provides a mathematical framework for understanding their intricate dance, allowing us to calculate pH based on the ratio of the buffer’s conjugate acid and base forms.
The protein buffer system stands out with its versatility and abundance, contributing significantly to intracellular pH stability. Unlike the bicarbonate buffer, which predominates in extracellular fluids, protein buffers are found both within cells and in the bloodstream.
In the blood, hemoglobin, the oxygen-carrying protein, plays a dual role as a pH regulator. It binds to protons when pH drops, and releases them when pH rises, stabilizing the pH despite changes in acidity.
Within cells, proteins with histidine residues, like hemoglobin, act as buffers. Histidine’s unique chemical nature allows it to accept or donate protons, fine-tuning the pH of the intracellular environment.
The protein buffer system, often overlooked in the face of the more renowned bicarbonate buffer, is nevertheless an indispensable player in maintaining pH stability. Its proteins, like tiny pH custodians, tirelessly patrol our cells and blood, ensuring that the delicate balance of life is preserved.
Hemoglobin: The Versatile Buffer in Blood and Cells
Hemoglobin, the renowned oxygen transporter in red blood cells, plays a versatile role in maintaining optimal pH levels within the body. This essential protein exists not just in the circulatory system but also within the cells, where it contributes significantly to intracellular pH stability.
In blood, hemoglobin acts as a mobile buffer. It can bind to hydrogen ions (H+) when blood pH levels drop, effectively neutralizing acidity and preventing pH from becoming too acidic. Conversely, when blood pH becomes too alkaline, hemoglobin can release H+ ions, counteracting alkalinity and restoring pH balance.
Within cells, hemoglobin’s buffering capacity is crucial for enzyme activity and optimal cellular function. Many enzymes involved in cellular metabolism require a specific pH range to function properly. Hemoglobin’s ability to bind and release H+ ions helps stabilize intracellular pH, ensuring that enzymes can continue their essential roles.
In summary, hemoglobin’s versatile buffering capabilities extend both to the circulatory system and to individual cells. By neutralizing acidity and alkalinity, hemoglobin helps maintain optimal pH levels, which is vital for the proper functioning of enzymes and overall cellular health.
Carbonic Anhydrase: The Enzyme That Powers the Bicarbonate Buffer System
In the intricate dance of maintaining acid-base balance within our bodies, carbonic anhydrase plays a starring role. This industrious enzyme lies at the heart of the bicarbonate buffer system, the body’s primary means of keeping blood pH tightly regulated.
The bicarbonate buffer system operates like a chemical symphony, orchestrating the conversion of carbon dioxide (CO2) into bicarbonate ions (HCO3-) and hydrogen ions (H+). This transformation, facilitated by carbonic anhydrase, ensures that pH fluctuations are kept to a minimum.
Carbonic anhydrase is a remarkable catalyst, accelerating the hydration of CO2 by a millionfold. Its presence in red blood cells and the lining of the lungs enables the rapid conversion of CO2 produced by cellular respiration into H+ and HCO3-. This process, known as the “plasma chloride shift,” plays a crucial role in regulating blood pH.
The impact of carbonic anhydrase extends beyond blood acid-base balance. It also contributes to maintaining pH equilibrium within cells, working in concert with intracellular buffers to ensure optimal conditions for enzyme activity and cellular function.
The significance of carbonic anhydrase is further underscored by its role in the Henderson-Hasselbalch equation, a mathematical tool used to calculate pH. This equation takes into account the concentrations of both the weak acid (H2CO3) and its conjugate base (HCO3-) in the presence of carbonic anhydrase, providing a precise measure of pH in various buffer systems, including the bicarbonate system.
In summary, carbonic anhydrase is the driving force behind the bicarbonate buffer system, enabling the body to maintain acid-base balance within a narrow range. Its impact on pH equilibrium extends from the blood to intracellular compartments, ensuring optimal conditions for physiological processes to thrive.
The Delicate Dance of Acid-Base Balance
Our bodies are incredible ecosystems, maintaining a delicate balance of physical and chemical processes. One crucial aspect of this harmony is acid-base balance, which ensures that the pH levels in our blood and cells remain within a narrow range. This intricate dance is orchestrated by a team of “buffer systems,” each playing a vital role in keeping our pH stable.
Imagine your body as a bustling city, each system like a department working in unison to keep things running smoothly. First, we have the bicarbonate buffer system, the primary watchdog for blood pH. Like a vigilant traffic controller, it swiftly converts carbon dioxide (CO2) into hydrogen ions (H+) and bicarbonate ions (HCO3-), maintaining a harmonious equilibrium.
But our body has backups! The phosphate buffer system and protein buffer system are like diligent assistants, ready to step in and stabilize pH when needed. Proteins, the workhorses of our cells, also contribute to this delicate balance.
In the spotlight of these buffer systems shines hemoglobin, a superhero with a double life. Found in red blood cells, it not only transports oxygen, but also acts as a buffer, ensuring optimal pH for enzyme activity.
But the unsung hero of the acid-base dance is carbonic anhydrase, an enzyme that accelerates the conversion of CO2 to H+ and HCO3-. Without this maestro, our buffer systems would lose their rhythm.
Maintaining acid-base balance is like conducting a delicate orchestra. The bicarbonate, phosphate, and protein buffer systems, hemoglobin, and carbonic anhydrase work in harmony to keep our pH in check. This intricate collaboration ensures that our cells have the optimal pH for proper enzyme function and cellular health. It’s a testament to the incredible design and resilience of our bodies.
The Henderson-Hasselbalch Equation: A Key Player in pH Regulation
In the realm of biochemistry, maintaining a stable pH balance is paramount for the proper functioning of cellular processes. Buffer systems play a crucial role in this pH regulation, and the Henderson-Hasselbalch equation serves as a valuable mathematical tool for understanding and calculating pH changes within these systems.
The Henderson-Hasselbalch equation, developed by Lawrence J. Henderson and Karl A. Hasselbalch, provides a mathematical relationship between the pH of a buffer solution and the concentrations of its weak acid and conjugate base components:
pH = pKa + log([A-] / [HA])
In this equation:
- pH represents the acidity or alkalinity of the solution.
- pKa is the acid dissociation constant of the weak acid, a measure of its strength.
- [A-] is the concentration of the conjugate base.
- [HA] is the concentration of the weak acid.
Understanding the Henderson-Hasselbalch Equation
The equation suggests that the pH of a buffer solution is directly related to the ratio of conjugate base to weak acid concentrations. When the ratio of conjugate base to weak acid is high, the pH will be higher (less acidic), indicating a more alkaline solution. Conversely, when the ratio is low, the pH will be lower (more acidic).
The pKa value provides a benchmark for the pH at which the concentrations of conjugate base and weak acid are equal. At the pKa, the pH of the solution is equal to the pKa, and the buffer is at its maximum buffering capacity.
The Henderson-Hasselbalch equation allows us to calculate the pH of a buffer solution when the concentrations of the weak acid and conjugate base are known. It also enables us to predict how the pH will change when either the acid or conjugate base is added to the solution.
Applications of the Henderson-Hasselbalch Equation
The Henderson-Hasselbalch equation has numerous applications in various buffer systems within the body, including:
- Bicarbonate buffer system: Regulates the pH of blood and other body fluids.
- Phosphate buffer system: Maintains the pH of intracellular fluids.
- Protein buffer system: Contributes to pH stability in proteins and other biological molecules.
The Henderson-Hasselbalch equation is a powerful tool for understanding and predicting pH changes in buffer systems. It provides a quantitative framework for analyzing these systems and their role in maintaining a stable pH balance, which is essential for cellular function and overall health.