Elements And Compounds: The Foundation Of Chemical Bonding And Matter
Elements, the fundamental building blocks of matter, and compounds, substances formed by chemical bonding, are intricately related. Elements, with their unique atomic structures, serve as the basis for compound formation. Chemical bonding, including covalent, ionic, and metallic bonding, unites atoms to form compounds with distinct properties that differ from their elemental components. This interplay between elements and compounds is crucial in shaping the chemical world, enabling the formation of countless substances with diverse applications and functions.
- Definitions of elements and compounds
- Overview of their relationship
Elements and Compounds: The Fundamental Building Blocks of Chemistry
In the realm of chemistry, understanding the relationship between elements and compounds is like embarking on an enchanting journey filled with tiny building blocks and countless possibilities. Let’s unravel the captivating tale that connects these two fundamental concepts.
Elements: The Alphabet of the Chemical World
Imagine the periodic table as a vibrant tapestry woven with the threads of elements, the simplest substances that cannot be chemically broken down any further. Each element, like a unique character with its quirks and traits, is represented by a symbol. For instance, hydrogen (H) is the lightest element, while uranium (U) is renowned for its heavy weight and radioactive nature.
These elements are organized in a systematic arrangement, capturing their chemical properties and relationships. The periodic table is a roadmap to the building blocks of our universe, guiding us through the intricate dance of matter.
Compounds: When Elements Dance
While elements stand alone as individuals, compounds emerge when two or more elements join forces, creating substances with entirely new characteristics. These chemical unions form the compounds that make up the world around us.
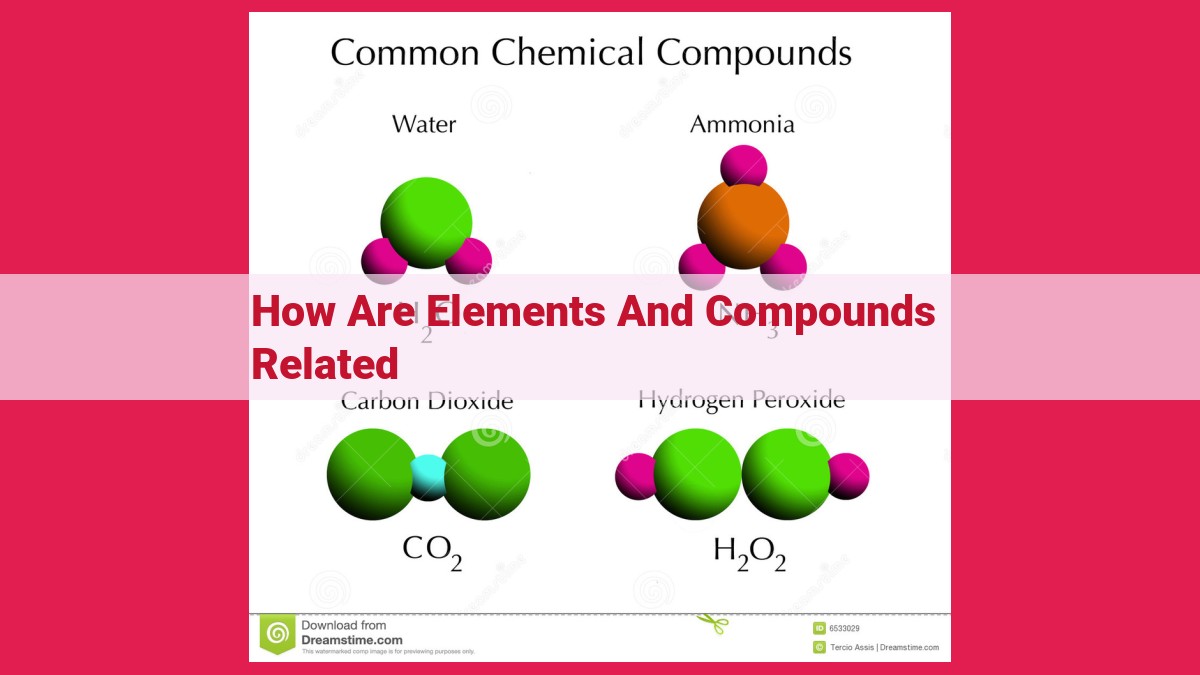
Take water (H2O), a compound that quenches our thirst and sustains life. It’s a symphony of two hydrogen atoms and one oxygen atom, harmoniously intertwined through covalent bonding. This bonding occurs when atoms share electrons, creating a molecular embrace.
The Interplay of Elements and Compounds
The relationship between elements and compounds is a dynamic interplay, akin to a captivating ballet. Elements provide the raw material, the building blocks, while compounds are the exquisite creations that result from their chemical dance.
The type of bonding between atoms determines the properties of the resulting compound. For instance, ionic bonding (electron transfer) leads to compounds with high melting points, like salt (NaCl), while metallic bonding (metal atom interactions) results in compounds that conduct electricity well, like copper.
The journey of elements and compounds is a testament to the power of chemistry. By understanding how these building blocks interact, we gain insights into the intricate fabric of the natural world. From the water we drink to the medicine that heals us, elements and compounds shape our lives in countless ways.
Elements: The Building Blocks of Matter
The Periodic Table: A Symphony of Elements
Picture a grand orchestra, with each instrument representing an element. The periodic table is a masterpiece of organization, categorizing these elements based on their atomic structure. Hydrogen, the lightest, sits at the top left, while uranium, the heaviest, resides at the bottom right. This table serves as a roadmap, guiding us through the vast landscape of elements.
Atomic Architecture: The Blueprint of Existence
At the heart of every element lies the atom. Imagine a tiny, spinning planet with a nucleus as its core. Protons, positively charged particles, reside in the nucleus, dictating the element’s identity. Neutrons, neutral particles, add stability to the nucleus. Finally, electrons, negatively charged particles, orbit the nucleus like stars around a black hole. The arrangement and number of these subatomic particles define each element’s unique fingerprint.
Isotopes: Variations on a Theme
Isotopes are like cousins within an element family. They share the same number of protons but differ in the number of neutrons. These variations influence the element’s properties, such as its mass and radioactivity. For instance, carbon exists as three isotopes: carbon-12, carbon-13, and carbon-14. Each isotope finds applications in different fields, from dating fossils to tracing metabolic pathways.
Compounds: Substances Formed by Chemical Bonding
In the realm of chemistry, elements – the fundamental building blocks of matter – take center stage as the key players in the formation of compounds, substances that arise through the captivating dance of chemical bonding. Understanding the intricate relationship between elements and compounds is crucial to unlocking the secrets of the chemical world.
One captivating facet of compounds is their molecular formation, the process by which atoms join forces to create new entities. When atoms collide, they can share or transfer electrons, leading to the formation of molecules, entities held together by covalent bonds. Covalent bonding involves the sharing of electron pairs, creating a strong and stable connection between the participating atoms.
Another remarkable phenomenon in compound formation is ion formation. In this process, atoms gain or lose electrons, transforming into electrically charged particles known as ions. Positively charged ions, or cations, result from electron loss, while negatively charged ions, or anions, arise from electron gain. The electrostatic attraction between ions drives the formation of ionic bonds, creating compounds with a crystalline structure.
Chemical reactions serve as the catalyst for compound formation and breakage. When elements or compounds come into contact, they undergo a chemical dance, exchanging electrons and rearranging their atomic structures. These reactions can lead to the creation of new compounds or the decomposition of existing ones. The outcome of a chemical reaction depends on a multitude of factors, including the types of reactants involved, their concentrations, and the presence of a catalyst.
Delving deeper into the world of compounds, we discover that the chemical bonding type profoundly influences their properties. Covalent bonds create molecules with distinct shapes and bond lengths, while ionic bonds give rise to crystalline compounds with high melting and boiling points. These differences in bonding are responsible for the vast array of compounds we encounter in nature and in our everyday lives.
Understanding the interplay between elements and compounds is essential for unraveling the complexities of our chemical universe. Elements serve as the raw materials for compound formation, while chemical bonding acts as the architect, shaping the structure and properties of these newly created substances. By delving into the enchanting realms of elements and compounds, we uncover the secrets behind the diverse world of matter that surrounds us.
Chemical Bonding: The Force that Unites Atoms
An Atomic Romance
In the world of chemistry, elements and compounds dance together in a captivating interplay, united by an invisible force known as chemical bonding. Elements, the fundamental building blocks of matter, are like solo artists, each with their own unique identity. Compounds, on the other hand, are the harmonious blends of two or more elements, their distinct personalities merging to create new substances with properties all their own.
The Glue that Holds Us Together: Covalent Bonding
Imagine two atoms, eager to share their unpaired electrons like lovers exchanging secrets. This act of electron sharing creates a covalent bond, a bond of intimacy between the atoms. Covalent bonds are the most common type of chemical bond, responsible for the formation of molecules, the microscopic building blocks of life.
The Exchange of Power: Ionic Bonding
In the world of chemistry, not all bonds are created equal. Some elements prefer to give up electrons like generous souls, while others are more eager to receive them. When an atom donates an electron to another, a positively charged cation is born, while the recipient transforms into a negatively charged anion. These oppositely charged ions attract each other like magnets, forming an ionic bond. Ionic bonds are the backbone of salts, compounds that dissolve in water to conduct electricity.
A Metallic Embrace: Metallic Bonding
In the realm of metals, a different kind of bonding takes hold. Metallic bonds arise from a sea of free-floating electrons that surround the metal atoms. These electrons are shared among all the atoms in the metal, creating a strong bond that holds the metal together like a united family. Metallic bonds give metals their characteristic properties, such as luster, malleability, and ductility.
The Interplay of Elements and Compounds
In the realm of chemistry, elements and compounds dance a harmonious waltz, each contributing its unique properties to the formation of myriad substances. Elements, the fundamental building blocks of matter, are found in their purest form on the periodic table. Each element boasts a distinct atomic number, denoting the number of protons in its nucleus, and a corresponding number of electrons that orbit the nucleus.
Compounds, on the other hand, are substances formed when elements chemically bond together. This chemical bonding is the key to unlocking the vast array of substances found in the world around us. The type of bonding determines the properties of the compound, resulting in a diverse spectrum of characteristics.
For instance, covalent bonding, where atoms share electrons, often creates molecular compounds with covalent bonds between nonmetallic elements. These compounds are typically volatile, meaning they readily vaporize, and flammable, making them essential components of fuels and solvents. On the other hand, ionic bonding, where electrons are transferred from one atom to another, forms ionic compounds with ionic bonds between a metal and a nonmetal. These compounds tend to be solid, brittle, and high-melting, making them useful as building materials, salts, and fertilizers.
The interplay between elements and compounds extends beyond their structural differences. The properties of compounds can vary drastically from those of their constituent elements. For example, sodium and chlorine, two highly reactive elements, combine to form sodium chloride (table salt), a stable and non-reactive compound. This transformation highlights the profound influence of chemical bonding in shaping the characteristics of compounds.
In summary, elements and compounds engage in an intricate dance that gives rise to the vast diversity of substances in our world. Chemical bonding, the force that unites atoms, plays a pivotal role in determining the properties of these substances, showcasing the fascinating interplay between the fundamental building blocks of matter.
Molecular Geometry: The Dance of Atoms
The world around us is composed of countless substances, from the air we breathe to the water we drink. These substances are made up of elements, the building blocks of matter. But elements rarely exist alone; they combine to form compounds, substances with unique properties that differ from their individual components.
The arrangement of atoms within a compound is crucial to its properties. This arrangement, known as molecular geometry, determines how the compound interacts with other molecules and influences its physical and chemical behaviors.
Common molecular geometries include linear, trigonal planar, tetrahedral, and octahedral. Each geometry arises from the number and arrangement of electron pairs surrounding the central atom.
Linear molecules, such as carbon dioxide (CO2), form when two electron pairs are arranged opposite each other, creating a straight line. Trigonal planar molecules, like BF3, have three electron pairs arranged around the central atom, forming a flat triangle.
Tetrahedral molecules, such as CH4, possess four electron pairs arranged in a three-dimensional tetrahedron shape. Octahedral molecules, like SF6, have six electron pairs surrounding the central atom, forming an octahedron with eight faces.
Molecular geometry plays a pivotal role in the interactions between molecules. Linear molecules can pack tightly together, while branched molecules have more space between them. Trigonal planar molecules are polar, meaning they have a partial positive and negative charge, which affects their interactions with other molecules.
Understanding molecular geometry is essential for comprehending the behavior and properties of countless substances. It helps us predict their reactivity, solubility, and other characteristics that govern our everyday lives. From the materials we use to build our homes to the medicines that heal our ailments, molecular geometry shapes our world in innumerable ways.