Cesium: A Highly Reactive Alkali Metal With A Lone Valence Electron
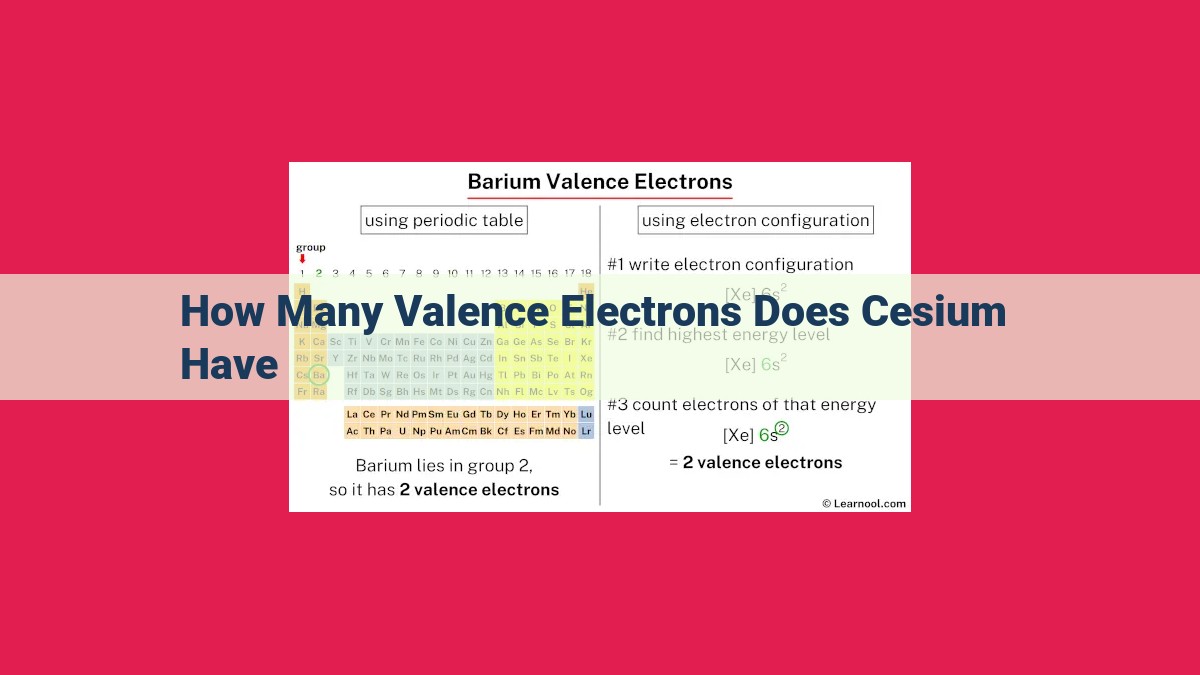
Cesium, with an atomic number of 55, has 55 protons in its nucleus, indicating 55 electrons overall. Its electron configuration, [Xe]6s1, reveals one electron in the outermost energy level, designated as the valence electron. Valence electrons are crucial for chemical bonding, as they dictate an element’s reactivity. Cesium’s single valence electron makes it a highly reactive alkali metal, readily reacting with water and sensitive to air. Alkali metals like cesium are characterized by their one valence electron, which influences their chemical properties and applications, such as in batteries and fertilizers.
Unveiling the Secrets of Cesium: The Element with a Unique Atomic Identity
Prologue
In the vast tapestry of elements that make up our universe, cesium stands out with an intriguing atomic identity. Atomic number, a fundamental property, plays a pivotal role in defining the element’s characteristics and shaping its position in the periodic table.
The Significance of the Atomic Number
Cesium possesses an atomic number of 55, indicating the presence of 55 protons in its atomic nucleus. Protons, positively charged particles, define an element’s identity and determine its chemical properties. The number of protons in an atom also determines the number of electrons, negatively charged particles that surround the nucleus.
Unveiling the Electron Configuration
The electron configuration of cesium reveals the arrangement of electrons in its energy levels. Cesium has 55 electrons, distributed in energy levels according to the Aufbau principle. The outermost energy level, known as the valence shell, contains one electron.
The Reactivity of Valence Electrons
Valence electrons are responsible for an element’s chemical reactivity. Cesium, with its single valence electron, exhibits a high reactivity due to its tendency to lose this electron. This electron loss leads to the formation of positive ions, giving cesium its characteristic alkali metal properties.
Delving into the Electron Configuration of Cesium: A Journey into Atomic Structure
Cesium, with its unique atomic structure, holds a captivating story within its electron configuration. This enigmatic element, belonging to the alkali metal family, possesses an electron arrangement that unveils its chemical prowess and unique properties.
Each atom of cesium harbors 55 electrons, meticulously distributed across five distinct energy levels or shells. Like a celestial orchestra, these electrons dance around the nucleus, each contributing to the element’s overall character.
The first energy level, closest to the nucleus, holds two electrons, representing the foundation of cesium’s atomic structure. The second energy level, slightly further out, accommodates eight electrons, forming a stable core. The third energy level witnesses the presence of 18 electrons, creating a more dynamic and reactive zone. The fourth energy level hosts another 18 electrons, adding to the element’s complexity. Finally, the fifth energy level, the outermost shell, proudly displays a single electron, a lone wanderer that dictates cesium’s chemical destiny.
This intricate arrangement of electrons defines cesium’s valence electron count, a crucial factor governing its chemical reactivity. Valence electrons, the outermost residents of an atom, determine how it interacts and forms bonds with other elements. In cesium’s case, its solitary valence electron yearns for companionship, making it highly reactive and eager to engage in chemical reactions.
Cesium’s electron configuration not only governs its reactivity but also reveals its position within the periodic table. As the last element in Group 1 (alkali metals), cesium’s electron configuration mirrors its chemical behavior. All alkali metals share a common trait: they possess a single valence electron, making them highly reactive and essential players in various chemical processes.
Understanding the electron configuration of cesium is not merely an academic pursuit. It unlocks a deeper comprehension of this element’s properties, its role in shaping the periodic table, and its significance in a myriad of scientific and technological applications.
Valence Electrons: The Key to Chemical Bonding
- Define valence electrons and explain their critical role in chemical bonding. Discuss how the number of valence electrons affects an element’s reactivity.
Valence Electrons: The Gateway to Chemical Connections
In the realm of chemistry, the concept of valence electrons holds immense significance. Valence electrons are the outermost electrons of an atom, dancing around the atom’s nucleus. They determine the atom’s chemical reactivity and ability to form bonds with other atoms.
Think of valence electrons as the sociable butterflies of the atomic world. They are the ones who venture out to interact with their neighbors, eager to make connections and form chemical alliances. The number of valence electrons an element possesses dictates its chemical personality and influences how it behaves in the great symphony of reactions.
For instance, sodium, with its single valence electron, is a gregarious fellow, always seeking a dance partner. It readily gives up its valence electron to form bonds, creating compounds such as sodium chloride (common salt). In contrast, helium, with its two valence electrons tightly bound to each other, is a loner. It forms bonds reluctantly, preferring to keep its valence electrons close to home.
The number of valence electrons also affects an element’s reactivity. Elements with more valence electrons tend to be more reactive, as they have a greater propensity to form bonds. This explains why alkali metals, like sodium and potassium, are highly reactive, while noble gases, like helium and argon, are chemically inert.
Chemical Properties of Cesium: A Reactive Alkali Metal
- Highlight the highly reactive nature of cesium as an alkali metal. Describe its reaction with water and its sensitivity to air.
Chemical Properties of Cesium: A Reactive Alkali Metal
Cesium, an element found in the periodic table, is known for its exceptional reactivity, making it one of the most volatile metals known to humankind. Its chemical properties are a testament to its unique atomic structure and valence electrons.
High Reactivity: A Nature’s Dance
Cesium belongs to the alkali metal group, characterized by the presence of a single valence electron in their outermost energy level. This lone electron yearns for a way out, eager to bond with other atoms, granting cesium an extreme proclivity to react.
Reaction with Water: A Symphony of Hiss and Flames
When introduced to water, cesium undergoes an impulsive and fiery reaction. The water molecules eagerly donate their electrons to cesium’s outstretched hand, forming hydrogen gas that erupts with a dramatic hiss. The heat released during this reaction can be so intense that it ignites the hydrogen gas, creating an eye-catching display of flames dancing atop the water’s surface.
Sensitivity to Air: A Royal’s Aversion to Commoners
Cesium’s vulnerability extends beyond its encounters with water. Its delicate nature makes it highly sensitive to air. The oxygen molecules in the atmosphere are equally eager to join cesium’s merry dance of electrons. This reaction forms an oxide layer on cesium’s surface, rendering it tarnished and dull.
The chemical properties of cesium paint a vivid portrait of a highly reactive and volatile element. Its single valence electron, like a restless adventurer, drives its relentless pursuit of chemical bonds. From its spectacular reaction with water to its sensitivity to air, cesium serves as a fascinating example of the intricate interplay between atomic structure and chemical reactivity.
Group 1 Elements: The Alkali Metals
Embrace the fascinating world of chemistry as we embark on an adventure into the realm of alkali metals, the vibrant family of elements nestled in Group 1 of the periodic table. With our focus on their captivating characteristics, we’ll unveil their secrets and explore their remarkable impact on our daily lives.
Common Characteristics
United by their innate generosity, alkali metals readily donate their one valence electron, making them highly reactive and eager to form bonds. This lone electron gives them a distinctive identity, defining their chemistry and setting them apart from their element counterparts.
Reactivity Unveiled
Like eager explorers venturing into uncharted territories, alkali metals embrace reactivity with fervor. They readily react with water, releasing an abundance of energy in dazzling displays of fireworks-like flames. This volatile nature makes them sensitive to air, requiring careful handling and storage under protective atmospheres.
Alkali metals, with their distinctive chemistry and captivating reactivity, serve as a testament to the boundless wonders of the periodic table. Their unique properties have carved a niche for them in various industrial applications, from illuminating our homes to fueling our vehicles. As we continue our journey through the annals of chemistry, let us delve deeper into the stories of these alkali metals, unlocking their secrets and unraveling their significance in our modern world.
Alkali Metals in Action: Applications and Importance
In the fascinating world of chemistry, alkali metals are renowned for their highly reactive nature. These elements, nestled in Group 1 of the periodic table, possess a solitary valence electron that eagerly participates in chemical reactions. This unique feature translates into an array of versatile applications, spanning from everyday products to cutting-edge technologies.
One of the most well-known uses of alkali metals is in batteries. The lithium-ion battery, a cornerstone of modern electronics, harnesses the electrochemical properties of lithium to store and release energy. These batteries power countless devices, from smartphones and laptops to electric vehicles, revolutionizing our daily lives.
Fertilizers represent another important application. Potassium, an alkali metal, plays a crucial role in plant growth and development. Potassium fertilizers replenish the soil with this essential nutrient, enhancing crop yields and ensuring food security for a growing global population.
Pharmaceuticals also benefit from the unique properties of alkali metals. Cesium chloride, a salt of cesium, is used in diagnostic imaging to help visualize organs and tissues. Lithium, another alkali metal, is prescribed as a medication to treat conditions such as bipolar disorder.
Beyond these specific applications, alkali metals also contribute to various industries. Sodium is used in the production of glass, paper, and detergents. Potassium is essential for the manufacture of soap and fertilizers. Rubidium finds use in atomic clocks, while francium is employed in cancer research.
The versatility of alkali metals stems from their highly reactive nature. Their eagerness to form chemical bonds makes them valuable reagents in various industrial processes. However, this reactivity also demands careful handling, as alkali metals can react explosively with water and air.
Understanding the properties and applications of alkali metals is crucial for unlocking their potential. The knowledge gained from ongoing research and development in this field will undoubtedly lead to even more groundbreaking applications in the future.
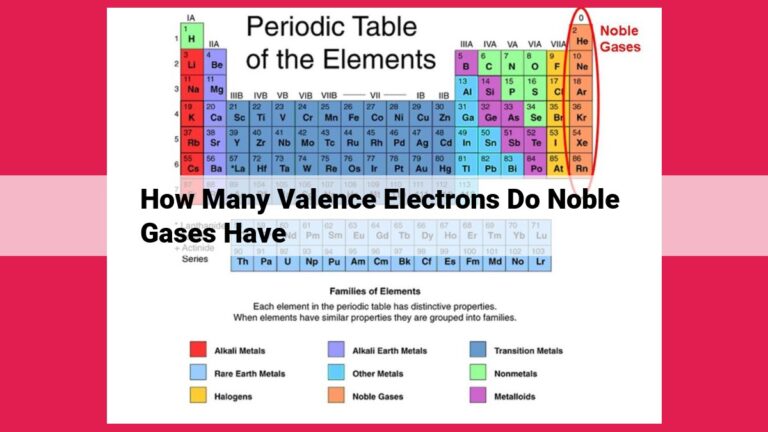
Noble Gases: The Stable Cousins of Alkali Metals
In the realm of chemistry, elements dance in a delicate balance of reactivity. Alkali metals, like cesium, burst into action, eagerly shedding their valance electron to form bonds. Contrastingly, noble gases stand as the epitome of stability, their electron configurations perfectly filled, leaving them inert.
Noble gases occupy Group 18 of the periodic table, the epitome of stability. Helium, neon, argon, krypton, xenon, and radon—these elements share a common characteristic: their outermost energy level is complete, bestowing upon them an air of indifference. Unlike their reactive alkali metal cousins, noble gases have no desire to participate in chemical reactions.
This peculiar stability stems from the octet rule: atoms strive to achieve a stable electron configuration of eight valence electrons. Noble gases have already attained this coveted state, leaving them perfectly content in their own solitude. They lack the drive to either gain or lose electrons, making them the ultimate loners of the chemical world.
Their inert nature finds practical applications in various fields. Helium, for instance, is used in balloons and airships due to its low weight and non-flammability. Neon lights up our streets and signs, while argon shields welding operations from the atmosphere. Even the radioactive radon has a role in detecting underground uranium deposits.
The contrast between alkali metals and noble gases highlights the diversity of elements. While one group eagerly engages in chemical reactions, the other stands aloof, a testament to the intricate interplay of electron configurations and chemical behavior. The periodic table, with its rows and columns of elements, serves as a valuable guide, unraveling the secrets of each element’s character.
The Periodic Table: Your Guide to Understanding the Elements
In the realm of chemistry, the periodic table reigns supreme as an invaluable tool for comprehending the myriad of elements that make up our universe. It’s like a meticulously organized encyclopedia of elements, arranging them in a way that reveals their profound secrets.
The Secret Code: Electron Configuration
The periodic table is not merely a random assortment of elements; it’s a tapestry woven together by the intricate patterns of electron configuration. Each element’s atomic number – the number of protons in its nucleus – dictates the number of electrons it possesses. These electrons, in turn, occupy specific energy levels around the nucleus.
The arrangement of electrons in these energy levels is like a unique fingerprint for each element, determining its chemical behavior. Elements with similar electron configurations tend to have similar properties, creating distinct groups within the periodic table.
Group 1: The Alkali Metals
Group 1 elements, known as the alkali metals, occupy the far left column of the periodic table. Their defining characteristic is their lone valence electron – an electron in the outermost energy level that eagerly participates in chemical reactions. This single valence electron makes alkali metals highly reactive, readily combining with other elements to form ions.
Alkali Metals in Action: A Symphony of Applications
Alkali metals play a crucial role in various industries due to their reactivity. They serve as essential components in batteries, enhancing their energy storage capabilities. In fertilizers, they provide vital nutrients for plant growth. Even in the realm of medicine, alkali metals find their niche in pharmaceuticals, contributing to the development of life-saving medications.
Noble Gases: The Inert Aristocrats
In stark contrast to the reactive alkali metals, noble gases reside on the opposite side of the periodic table, maintaining their aloof presence in Group 18. These elements have a full complement of electrons in their outermost energy level, rendering them exceptionally stable and unreactive. Their inert nature has earned them the title “noble gases.”
The Periodic Table: A Window into the Elemental World
The periodic table is more than just a static chart; it’s a dynamic tool that empowers us to predict the properties and behavior of elements based on their electron configuration. Its patterns and relationships reveal the interconnectedness of the elements, providing a deeper understanding of the complexities of our chemical world.