Optimize Cellular Ph For Enzyme Activity And Cell Function: A Guide To Homeostasis
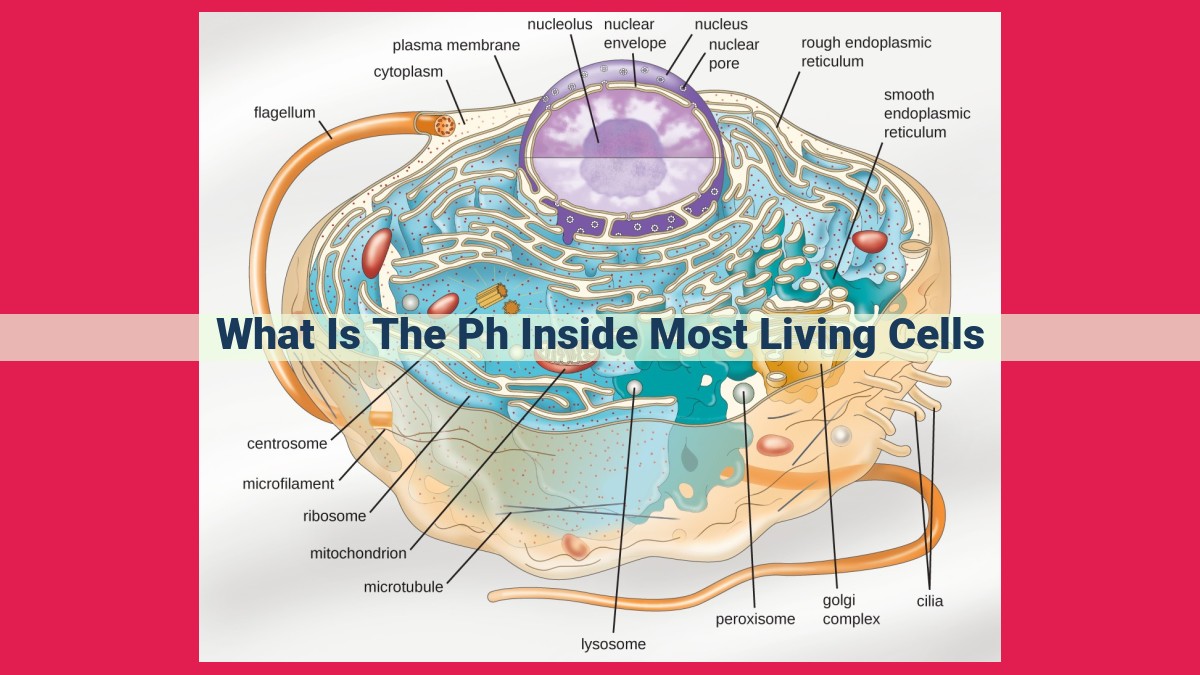
The pH inside most living cells is typically around 7.2-7.4, which is slightly alkaline. This optimal pH range is crucial for enzyme activity, protein structure, and proper cell function. Cytoplasmic pH is regulated by various mechanisms, including buffers, ion transporters, and the sodium-potassium pump. Buffers, such as the bicarbonate buffer system, neutralize acids and bases to maintain a stable pH. Maintaining optimal pH is essential for homeostasis, as pH changes can disrupt cellular processes and lead to dysfunction.
Understanding pH: A Guide to Chemical Balance in Biological Systems
In the intricate tapestry of life, maintaining a delicate chemical balance is crucial. One key aspect of this balance is the regulation of pH, a measure of the acidity or basicity of a solution. pH plays a critical role in a wide range of biological processes, from enzyme activity to cellular function.
pH: A Measure of Acidity and Basicity
The pH of a solution is measured on a scale of 0 to 14, with 0 being the most acidic and 14 being the most basic. A pH of 7 represents neutrality, meaning the solution contains equal concentrations of hydrogen ions (H+) and hydroxide ions (OH-).
Importance of pH in Biological Systems
pH is essential for the proper functioning of living organisms. Most biological processes operate within a narrow pH range. For example, pH affects enzyme activity, protein stability, and nerve impulse transmission. Deviations from the optimal pH range can disrupt these processes and lead to cellular dysfunction or even cell death.
Buffers: Guardians of pH Stability
To maintain the delicate balance of pH, organisms employ substances called buffers. Buffers are mixtures of weak acids and their conjugate bases or weak bases and their conjugate acids. When a small amount of acid or base is added to a buffered solution, the buffer system neutralizes the added ions, preventing drastic changes in pH.
Sodium-Potassium Pump: A Cellular pH Regulator
The sodium-potassium pump is a membrane-bound protein that helps maintain pH balance in cells. It transports sodium ions (Na+) out of the cell and potassium ions (K+) into the cell. This process creates an electrochemical gradient across the cell membrane, which influences the movement of other ions, including H+.
Intracellular pH: The Vital Balance Within
Every cell in our bodies operates within a delicate pH balance, a crucial factor for maintaining cellular health and function. Intracellular pH, specifically, refers to the pH level within the cell’s cytoplasm. Understanding this pH balance and its regulation is essential for comprehending cellular physiology.
Optimal Range and Importance
Intracellular pH must be maintained within a narrow optimal range to ensure cellular processes function smoothly. The ideal pH for most cells is slightly alkaline, around 7.2. Deviations from this range can disrupt vital cellular activities such as enzyme activity, protein synthesis, and cell metabolism.
Cytoplasmic pH and its Regulation
Maintaining the correct cytoplasmic pH is a dynamic process involving several mechanisms. Cells primarily use buffer systems to neutralize acid or base inputs and ion transporters to regulate the exchange of ions across the cell membrane.
Mechanisms to Maintain Intracellular pH
1. Buffer Systems:
Buffers are substances that help resist changes in pH by absorbing or releasing ions. Intracellular buffers include proteins, phosphates, and bicarbonate. The bicarbonate buffer system plays a pivotal role in maintaining cytoplasmic pH stability.
2. Ion Transporters:
Ion transporters, such as the sodium-potassium pump, help regulate the influx and efflux of ions across the cell membrane. This exchange can indirectly influence intracellular pH by consuming or generating hydrogen ions.
Role of Buffers in Cytoplasmic pH Stability
Buffers play a critical role in maintaining cytoplasmic pH stability by acting as a first line of defense against acid-base disturbances. They can rapidly neutralize small fluctuations in pH, preventing significant deviations from the optimal range.
Intracellular pH is a meticulously controlled aspect of cellular physiology. By maintaining the correct pH balance, cells ensure the proper functioning of vital processes and optimal cellular health. Understanding the mechanisms that regulate intracellular pH is essential for comprehending cellular biology and the implications of pH imbalances in various diseases.
Homeostasis: Maintaining the Delicate Balance of pH
The Significance of Intracellular pH Control
The pH level within our cells is crucial for a myriad of biological processes. It affects enzyme activity, protein stability, and even the distribution of ions across cell membranes. Maintaining optimal intracellular pH is essential for cellular function and overall health.
Physiological Mechanisms for pH Regulation
The body employs several mechanisms to regulate pH, including:
- Respiratory Regulation: The lungs control carbon dioxide levels, which in turn affects blood pH. When blood pH drops, the respiratory rate increases to exhale more carbon dioxide and raise pH.
- Renal Regulation: The kidneys filter waste products from the blood and can adjust bicarbonate levels to influence pH. When pH is low, the kidneys excrete more acid and retain more bicarbonate, raising pH.
- Buffering Systems: Buffers are molecules that neutralize acids and bases, preventing drastic pH changes. Bicarbonate and phosphate buffers play crucial roles in maintaining intracellular pH stability.
How Buffers Help Buffer Acid-Base Disturbances
Buffers work by accepting or releasing protons to neutralize pH changes. For example, when excess acid is added to a buffer solution, the buffer reacts to form a weaker acid and release protons, thereby lowering the overall acidity. Conversely, when excess base is added, the buffer forms a weaker base and consumes protons, raising the pH.
Homeostatic Responses to pH Changes
When pH levels deviate from the optimal range, homeostatic responses occur to restore balance.
- Acidosis: If pH drops (becomes more acidic), the body responds by increasing respiratory rate and retention of bicarbonate.
- Alkalosis: If pH rises (becomes more basic), the body responds by decreasing respiratory rate and excretion of bicarbonate.
Maintaining optimal intracellular pH is vital for cellular health and function. The body employs a combination of respiratory, renal, and buffering mechanisms to regulate pH levels and respond to acid-base disturbances. Understanding the importance of pH homeostasis helps us appreciate the complexity and resilience of human physiology.
Buffers: Key Players in pH Regulation
- Bicarbonate buffer system
- Importance of carbon dioxide and bicarbonate in pH buffering
- How buffers help maintain intracellular pH
Buffers: The Unsung Heroes of pH Regulation
In the tapestry of life, pH plays a pivotal role, orchestrating countless biological processes with exquisite precision. Yet, maintaining optimal pH can be a daunting task in the face of constant chemical fluctuations. Enter buffers, the unsung heroes that safeguard our cells by soaking up excess acids or bases, ensuring a harmonious balance.
One of the most crucial buffer systems in our bodies is the bicarbonate buffer system. This dynamic duo, comprising carbon dioxide (CO2) and bicarbonate ions (HCO3-), works diligently to buffer both intracellular and extracellular pH levels.
CO2, a byproduct of cellular respiration, plays a key role in buffering. When blood pH drops below 7.35, an enzyme called carbonic anhydrase catalyzes the conversion of excess CO2 and water into HCO3-. This reaction effectively absorbs protons (H+), raising pH. Conversely, when blood pH rises above 7.45, HCO3- is converted back into CO2 and water, releasing protons and lowering pH.
The bicarbonate buffer system is also instrumental in maintaining intracellular pH. Cells meticulously control their pH within a narrow range, typically between 6.8 and 7.2, using a variety of mechanisms. Buffers, including bicarbonate, play a crucial role in stabilizing cytoplasmic pH.
The sodium-potassium pump, a vital membrane protein, also contributes to pH homeostasis. This pump exchanges sodium ions (Na+) for potassium ions (K+), creating a pH gradient across the cell membrane. The resulting electrical gradient helps drive important cellular processes, such as nutrient transport and ATP synthesis.
In conclusion, buffers, like the bicarbonate buffer system, work tirelessly behind the scenes to maintain optimal pH. By absorbing excess acids or bases, they protect cells from harmful pH fluctuations, ensuring the smooth functioning of biological processes. Their role in intracellular pH regulation and the sodium-potassium pump further underscores their significance in the intricate symphony of life.
The Sodium-Potassium Pump and pH: A Balancing Act
In the realm of cells, maintaining a stable internal environment is paramount for life. pH, a measure of acidity or alkalinity, plays a crucial role in this equilibrium. One key player in pH regulation is the sodium-potassium pump, a molecular machinery that orchestrates a delicate dance of ions across cell membranes.
The Sodium-Potassium Pump: An Ion Transporter
The sodium-potassium pump, also known as the Na+/K+ ATPase, is a transmembrane protein that actively transports sodium (Na+) ions out of cells and potassium (K+) ions into cells. This seemingly simple process has profound implications for cellular function.
pH’s Influence on Pump Activity
pH, the measure of hydrogen ion concentration, exerts a significant influence on the activity of the sodium-potassium pump. When intracellular pH decreases, becoming more acidic, the pump’s activity increases in an attempt to restore pH balance. Conversely, when intracellular pH increases, becoming more alkaline, pump activity slows down.
Implications for Cellular Function
The pH-dependent regulation of the sodium-potassium pump has far-reaching implications for cellular function. For instance, nerve cell activity relies heavily on the proper operation of the sodium-potassium pump. Changes in pH can disrupt nerve signaling, leading to a cascade of physiological effects. Similarly, the sodium-potassium pump plays a crucial role in maintaining the resting potential of cells, which is essential for normal cell function.
In conclusion, the sodium-potassium pump is an indispensable component of cellular pH regulation. Its activity is carefully modulated by pH, ensuring that cells can maintain a stable internal environment and function optimally.