Cations: Positively Charged Ions And Their Impact In Science
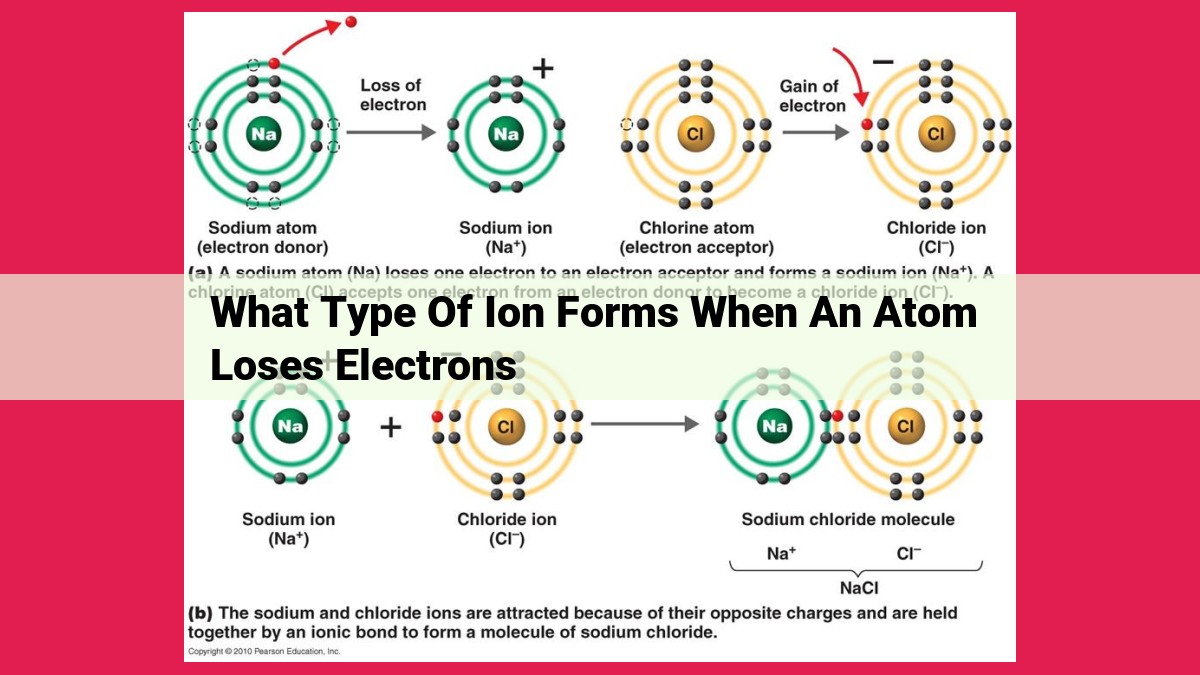
When an atom loses electrons, it forms a positively charged ion known as a cation. The number of lost electrons determines the charge of the cation, with each lost electron adding a +1 charge. Cations are formed when atoms lose their valence electrons, the outermost electrons that participate in chemical reactions. Common cations include sodium (Na⁺), magnesium (Mg²⁺), and calcium (Ca²⁺). Cations play a crucial role in various fields, including chemistry, biology, and medicine, where they participate in ion exchange, pH regulation, and nerve signaling processes.
Dive into the World of Ions: Understanding Ion Formation
In the realm of chemistry, ions play a pivotal role in shaping the properties of matter and driving many reactions. To fully comprehend the wonders of ions, let’s embark on a journey to understand how they come into existence.
What are Ions?
Simply put, ions are electrified atoms or molecules that have lost or gained electrons, resulting in an uneven distribution of charge. This imbalance creates a magnetic field and unique chemical properties.
The Process of Ion Formation
When electrons escape from an atom or molecule, the resulting particle becomes positively charged and is known as a cation. On the other hand, when electrons enter the atomic structure, the particle becomes negatively charged and is called an anion. This electron exchange process is the essence of ion formation.
Cations: Positively Charged Ions
In the realm of chemistry, the formation of ions is a captivating dance of electrons and atoms. Among these charged particles, cations stand out as positively charged ions, carrying an aura of intrigue due to their intriguing nature.
Imagine an atom, a tiny universe in itself, teeming with electrons orbiting its nucleus. Normally, these electrons remain in a harmonious balance, their negative charge counteracting the positive charge of the nucleus. However, under certain circumstances, an atom may lose one or more electrons, shedding its neutral disposition and becoming a cation.
Electron Loss and Cation Charge
The departure of electrons from an atom has a profound impact on its electrical character. As electrons bid farewell, the atom’s positive charge becomes more dominant, leading to the formation of a cation. The number of electrons lost dictates the charge of the cation. For instance, if an atom loses one electron, it transforms into a monocation (cation with a +1 charge), while the loss of two electrons results in a dication (cation with a +2 charge).
Examples of Cations
The world of cations is vast and varied, with each element displaying its own unique propensity to form positively charged ions. Let’s delve into some common examples that play a vital role in various fields:
- Sodium (Na⁺): A monocation that readily forms when sodium atoms lose a single electron. It’s essential for maintaining fluid balance in the human body and plays a crucial role in nerve signaling.
- Magnesium (Mg²⁺): A dication that emerges when magnesium atoms shed two electrons. It contributes to bone health, muscle function, and the proper functioning of enzymes in our bodies.
- Calcium (Ca²⁺): Another dication that helps strengthen bones and teeth. It also plays a pivotal role in muscle contraction and blood clotting.
Significance of Cations
Cations are not mere bystanders in the grand scheme of chemistry. They actively participate in a wide range of processes, shaping the world around us:
- Ion Exchange: Cations are instrumental in processes like water softening, where they exchange places with unwanted ions to improve water quality.
- pH Regulation: Cations, particularly hydrogen ions (H⁺), play a critical role in maintaining the delicate pH balance of fluids, including our blood and oceans.
- Nerve Signaling: Cations, such as sodium and potassium, are the driving force behind nerve impulses, enabling communication between cells in our bodies.
Electron Loss and Ion Charge: The Impact on Ion Identity
Ions, fascinating chemical entities, arise when atoms undergo a transformation that alters their electron count. This loss or gain of electrons significantly impacts the ion’s charge and chemical behavior. In the case of positively charged ions, known as cations, the number of lost electrons plays a pivotal role in determining their identity and reactivity.
Understanding Electron Loss and Ion Charge
When an atom loses electrons, it acquires a positive charge due to an imbalance between its protons and electrons. The number of lost electrons directly influences the net charge of the resulting cation. Each lost electron leads to an increase in the cation’s charge by one unit, represented as a superscript plus sign (+) following the chemical symbol of the element.
Notation for Cation Charge
To accurately denote the charge of a cation, a simple notation system is used. The charge number, represented by a Roman numeral in parentheses, indicates the number of lost electrons. For example, sodium, after losing one electron, becomes a cation denoted as Na⁺, indicating a net charge of +1. Similarly, magnesium, after losing two electrons, forms Mg²⁺, with a net charge of +2.
Examples of Cations and Their Charges
Numerous elements readily form cations, exhibiting varying charge numbers based on the number of lost electrons. Here are a few common examples:
- Sodium (Na⁺): +1 charge
- Magnesium (Mg²⁺): +2 charge
- Calcium (Ca²⁺): +2 charge
- Aluminum (Al³⁺): +3 charge
- Iron (Fe³⁺): +3 charge
Implications for Cation Formation
The valence electrons of an atom, the outermost electrons occupying its highest energy level, greatly influence its tendency to form cations. Elements with few valence electrons tend to lose them easily, forming stable cations. For instance, sodium, with only one valence electron, readily forms Na⁺ to achieve a stable electron configuration.
The loss of electrons in ion formation is a fundamental process that shapes the identity and behavior of cations. Understanding the relationship between electron loss and ion charge is crucial for comprehending the chemistry and reactivity of ions. From their formation to their applications in diverse fields, ions play a vital role in our world.
Examples of Cations:
Cations, positively charged ions, are formed when atoms lose electrons. They play crucial roles in various fields like chemistry, biology, and medicine. Here are some common cations:
Sodium (Na⁺)
Sodium is a highly reactive alkali metal with one valence electron. When it loses this electron, it forms a sodium ion (Na⁺), becoming positively charged. Sodium ions are essential for maintaining fluid balance and nerve impulses in the body.
Magnesium (Mg²⁺)
Magnesium is an alkaline earth metal with two valence electrons. When it loses these electrons, it forms a magnesium ion (Mg²⁺), gaining a double positive charge. Magnesium ions are involved in muscle function, energy production, and bone health.
Calcium (Ca²⁺)
Calcium is another alkaline earth metal with two valence electrons. Upon losing these electrons, it forms a calcium ion (Ca²⁺). Calcium ions are crucial for bone strength, muscle contraction, and blood clotting.
Influence of Valence Electrons
The tendency of an atom to form cations is influenced by its valence electrons. Valence electrons are the electrons in the outermost shell of an atom, and they determine the atom’s chemical properties. Elements with fewer valence electrons tend to lose them easily, forming cations. For example, alkali metals like sodium have only one valence electron, making them highly likely to form cations.
Importance of Cations
Cations are essential for a wide range of biological processes and technological applications. In chemistry, they participate in ion exchange reactions and help regulate pH levels. In biology, they are involved in nerve signaling, muscle contraction, and bone formation. In medicine, cations are used in drugs and supplements to treat various conditions, such as electrolyte imbalances and nerve disorders.
The Vital Role of Cations in Chemistry, Biology, and Medicine
Beyond their fundamental significance in understanding atomic structure and chemical reactions, cations play crucial roles in various scientific disciplines. This article will delve into the practical applications of cations, exploring their indispensable functions in chemistry, biology, and medicine.
The Importance of Cations in Chemistry
Cations are essential for ion exchange reactions, which are widely used in water purification, wastewater treatment, and industrial processes. By exchanging positively charged ions with negatively charged ions, ion exchange resins remove impurities and regulate water quality.
Cations in Biology
Calcium ions (Ca²⁺), for example, are vital for muscle contraction, nerve impulse transmission, and bone formation. In addition, sodium ions (Na⁺) and potassium ions (K⁺) are critical for maintaining proper nerve function, fluid balance, and pH regulation.
The Role of Cations in Medicine
Cations find numerous applications in medicine. Lithium ions (Li⁺) are used to treat bipolar disorder, while magnesium ions (Mg²⁺) are employed to prevent eclampsia in pregnant women. Potassium chloride (KCl) is administered to correct electrolyte imbalances and treat cardiac arrhythmias.
Ion Exchange
Ion exchange is a process in which ions are exchanged between a solid phase (ion exchange resin) and a liquid phase (solution). This process is used in various applications, including:
- Water softening: Removing calcium and magnesium ions from water to make it less “hard.”
- Water purification: Removing impurities and contaminants from water.
- Industrial processes: Separating and concentrating specific ions.
pH Regulation
Cations can regulate pH by neutralizing acids and bases. The sodium-potassium pump in cell membranes uses Na⁺ and K⁺ ions to maintain the proper pH inside and outside of cells.
Nerve Signaling
Cations are involved in the transmission of nerve signals. When a nerve impulse is triggered, sodium ions flow into the neuron, causing depolarization. Subsequently, potassium ions flow out of the neuron, restoring its resting state.
Cations are not just theoretical concepts but play vital roles in various scientific disciplines. Their practical applications in fields such as chemistry, biology, and medicine underscore their significance in water treatment, body function regulation, and medical therapies. Understanding the importance of cations not only enhances our knowledge of the natural world but also provides insights for technological advancements and medical treatments.