The Role Of Carbonic Acid In Natural Phenomena: From Limestone Erosion To Geothermal Activity
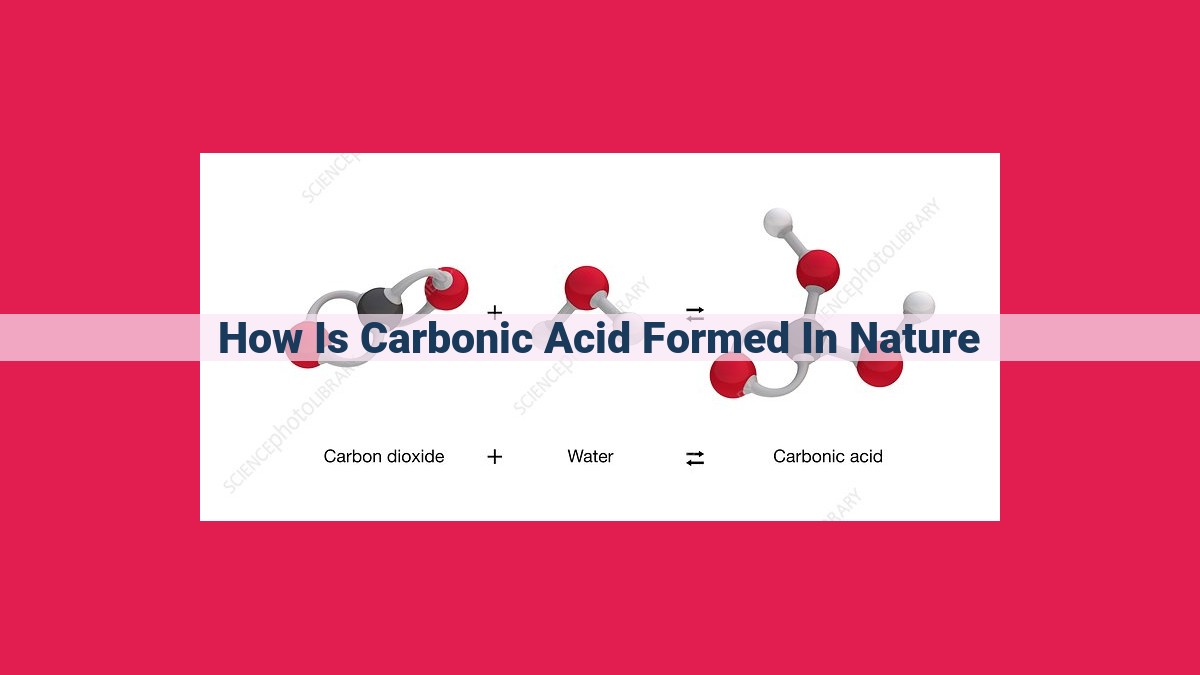
Carbonic acid forms naturally when carbon dioxide (CO2) dissolves in water (H2O), creating a weak acid (H2CO3). This process is influenced by temperature and pressure. Carbonic acid plays a crucial role in the weathering of limestone, contributing to the formation of karst landscapes. It is also essential for the formation of coral skeletons and the erosion that creates caves and subterranean wonders. Furthermore, carbonic acid is involved in geothermal activity, aiding in the separation of CO2 from groundwater, leading to geyser eruptions and hot springs.
Unveiling Carbonic Acid: A Keystone in Nature’s Symphony
Embark on a Journey into the Realm of Carbonic Acid
In the tapestry of natural phenomena, carbonic acid holds a pivotal place, playing a crucial role in shaping the world around us. Defined by its chemical formula, H2CO3, this unassuming substance unveils its significance as we explore its multifaceted nature.
The Alchemy of Water and CO2: The Birth of Carbonic Acid
The magic begins when carbon dioxide (CO2), a ubiquitous gas, encounters water. This union marks the birth of carbonic acid, a marvel of nature’s chemical alchemy. The interplay between CO2 and H2O, influenced by temperature and pressure, dictates the extent of this transformation.
Carbonic Acid’s Dance with Limestone: A Sculptural Romance
In the realm of geology, carbonic acid becomes an artistic maestro, sculpting masterpieces from limestone. It weasels its way into the pores of limestone, dissolving it and transforming it into calcium bicarbonate. This soluble form then embarks on a subterranean journey, ready to participate in the intricate choreography of stalactite and stalagmite formation.
Coral Reefs: A Vibrant Canvas Painted by Carbonic Acid
Beneath the shimmering seas, carbonic acid becomes an indispensable architect, crafting the intricate skeletons of coral. These skeletons, the foundation of majestic coral reefs, owe their existence to the interplay of carbonic acid and calcium carbonate. However, the shadows of ocean acidification loom large, threatening to disrupt this delicate balance.
Geothermal Wonders: Carbonic Acid’s Fiery Embrace
In the depths of the Earth, where geothermal energy reigns supreme, carbonic acid finds a fiery stage. It emerges as a catalyst in the separation of CO2 from heated groundwater, a process that gives rise to the awe-inspiring spectacle of geysers and the soothing embrace of hot springs.
Formation of Carbonic Acid through Water Dissolution
Water, the elixir of life, holds a hidden secret that plays a pivotal role in sculpting our planet’s landscapes and fostering life in its diverse ecosystems. This secret lies in the intriguing dance between carbon dioxide (CO₂) and water molecules. When these two elements collide, a remarkable transformation occurs, giving birth to a substance that holds immense significance – carbonic acid (H₂CO₃).
As CO₂ from the atmosphere embraces the vast expanse of water bodies, a subtle alchemy begins. The CO₂ molecules dissolve, merging seamlessly with water molecules. This intimate union triggers a series of chemical reactions, culminating in the formation of carbonic acid. The process unfolds in two distinct stages:
-
CO₂ Hydration: In the first stage, a single CO₂ molecule intertwines with a water molecule, forming carbonic acid (H₂CO₃). This reaction is relatively swift, occurring within milliseconds.
-
Hydrolysis: The carbonic acid molecule, however, is not content with its current state. It undergoes a further transformation, splitting into hydrogen ions (H⁺) and bicarbonate ions (HCO₃⁻). This dissociation reaction is gradual, taking several hours to complete.
The rate at which CO₂ dissolves in water and forms carbonic acid is influenced by several factors, including temperature and pressure. As temperature rises, the solubility of CO₂ in water diminishes, resulting in reduced carbonic acid formation. Conversely, increased pressure favors CO₂ dissolution and enhances carbonic acid production. These factors play a critical role in shaping the distribution and abundance of carbonic acid in various aquatic environments.
Carbonic Acid and Limestone: A Geological Symphony
The Limestone Enigma
Limestone, a sedimentary rock composed primarily of calcium carbonate, stands as a testament to the intricate interplay between water, carbon dioxide, and time. Its very formation is a story of transformation, where carbonic acid, the unsung hero, plays a pivotal role.
A Tale of Dissolution and Precipitation
As raindrops quench the thirst of the Earth, they dissolve carbon dioxide from the atmosphere, forming carbonic acid (H2CO3). This acidic solution, as it seeps through cracks and fissures in limestone, begins a process of chemical weathering, dissolving the calcium carbonate. The resulting calcium bicarbonate (Ca(HCO3)2) emerges as a soluble salt, carried by the flowing water.
The Rebirth of Limestone
As the subterranean journey progresses, conditions change. The pressure decreases, and the solubility of calcium bicarbonate declines. This shift triggers precipitation, where dissolved calcium carbonate recrystallizes and deposits on surfaces within the cave system. Layer by layer, these deposits build up, forming new limestone formations.
Karst Landscapes: A testament to Carbonic Acid’s Sculpting Power
The transformative power of carbonic acid extends beyond caves. Its persistent erosion shapes the landscape, creating unique geological formations known as karst. Sinkholes, underground rivers, and natural bridges are all products of carbonic acid’s relentless work, leaving behind a mesmerizing subterranean world.
Carbonic Acid and the Enigmatic Coral Reefs
- Highlight the importance of carbonic acid in the formation of coral skeletons.
- Discuss the threat of ocean acidification to coral health and reef ecosystems.
- Explain conservation efforts aimed at protecting coral reefs.
Carbonic Acid and the Enigmatic Coral Reefs
Carbonic acid plays a crucial role in the enchanting world of coral reefs. It’s like a magical elixir that nourishes these vibrant underwater cities. As carbon dioxide (CO2) dissolves in seawater, it forms carbonic acid, providing a source of the building blocks that corals need to construct their intricate skeletons. These skeletons form the foundation of coral reefs, creating a diverse and thriving ecosystem.
However, the ocean faces a growing threat known as ocean acidification. As atmospheric CO2 levels rise, so does the concentration of carbonic acid in the ocean. This can make it harder for corals to build and maintain their skeletons, putting these vibrant ecosystems at risk. The loss of coral reefs would have a devastating impact on marine biodiversity and the livelihoods of people who depend on them.
To combat this threat, scientists and conservationists are working together to protect these incredible underwater wonders. Coral restoration projects aim to repopulate damaged reefs, while research into ocean acidification helps us understand the challenges corals face and develop strategies to mitigate them. By raising awareness and advocating for ocean conservation policies, we can help ensure the future of these enchanting and essential ecosystems.
Carbonic Acid’s Architectural Feats: Caves and Subterranean Wonders
Beneath the surface of our Earth lies a hidden world of wonders, carved and shaped by the relentless erosion of carbonic acid. These subterranean sanctuaries, known as caves, are testament to the transformative power of this enigmatic compound.
Within these caves, water seeps through cracks and crevices, carrying with it dissolved carbon dioxide. This carbon dioxide reacts with the limestone bedrock, forming carbonic acid. As the carbonic acid relentlessly attacks the limestone, it slowly dissolves the rock, creating a network of intricate passageways.
Cave Formation
As carbonic acid seeps deeper into the limestone, it gradually widens the passageways, forming chambers and tunnels. Over time, these chambers may become interconnected, creating vast cave systems that can span kilometers. The shapes and sizes of caves depend on the character of the limestone, the amount of water available, and the time over which the erosion occurs.
Stalactites and Stalagmites
Inside caves, you’ll often encounter awe-inspiring formations called stalactites and stalagmites. Stalactites are cone-shaped structures that hang from the ceiling of caves. They form when water dripping from the ceiling dissolves limestone and deposits it as calcite on the roof.
Stalagmites, on the other hand, are cone-shaped structures that rise from the floor of caves. They form when water dripping from the roof evaporates, leaving behind calcite deposits. Over thousands of years, stalactites and stalagmites can grow towards each other, creating breathtaking columns that further enhance the allure of caves.
These subterranean wonders are a testament to the enduring power of carbonic acid. They offer a glimpse into the unseen forces that shape our planet and invite us to marvel at the beauty that lies hidden beneath our feet.
Geothermal Wonders: Unveiling the Secrets of Geysers and Hot Springs
Nestled amidst Earth’s enigmatic landscapes, geysers and hot springs captivate us with their spectacular displays and therapeutic allure. Their genesis lies in the depths of our planet, where an intricate dance between water, heat, and carbonic acid unfolds.
Carbonic acid, a product of dissolved carbon dioxide in water, plays a pivotal role in these geothermal wonders. As heated groundwater seeps through subterranean channels, it encounters carbon dioxide gas. This gas dissolves in the water, forming carbonic acid. The acidic solution then interacts with underground rock formations, primarily limestone.
The resulting chemical reaction releases more carbon dioxide, leading to a separation of gas from the groundwater. This process is akin to opening a bottle of soda, causing the release of bubbles. The accumulated carbon dioxide forms gas pockets, which build up pressure within the subterranean system.
When the pressure reaches a critical point, the gas pockets burst forth as geyser eruptions, propelling towering jets of water and steam into the air. The rhythmic and awe-inspiring eruptions of geysers, such as those in Yellowstone National Park, are a testament to the dynamic interplay between carbonic acid and geothermal forces.
In addition to geysers, carbonic acid also influences the formation of hot springs. As the carbonated groundwater seeps to the surface, it interacts with the surrounding rock, releasing dissolved minerals. These minerals, such as calcium carbonate, crystallize and accumulate over time, forming the characteristic terraced pools and otherworldly formations found in hot spring areas.
The therapeutic benefits associated with hot springs are attributed to the presence of dissolved minerals and gases, including carbonic acid. Immersion in hot spring waters can alleviate muscle pain, improve circulation, and promote relaxation. Thus, geothermal wonders, powered by carbonic acid, offer not only captivating natural spectacles but also potential health benefits.