Carbon: A Versatile Element With Six Electrons In Three Energy Levels
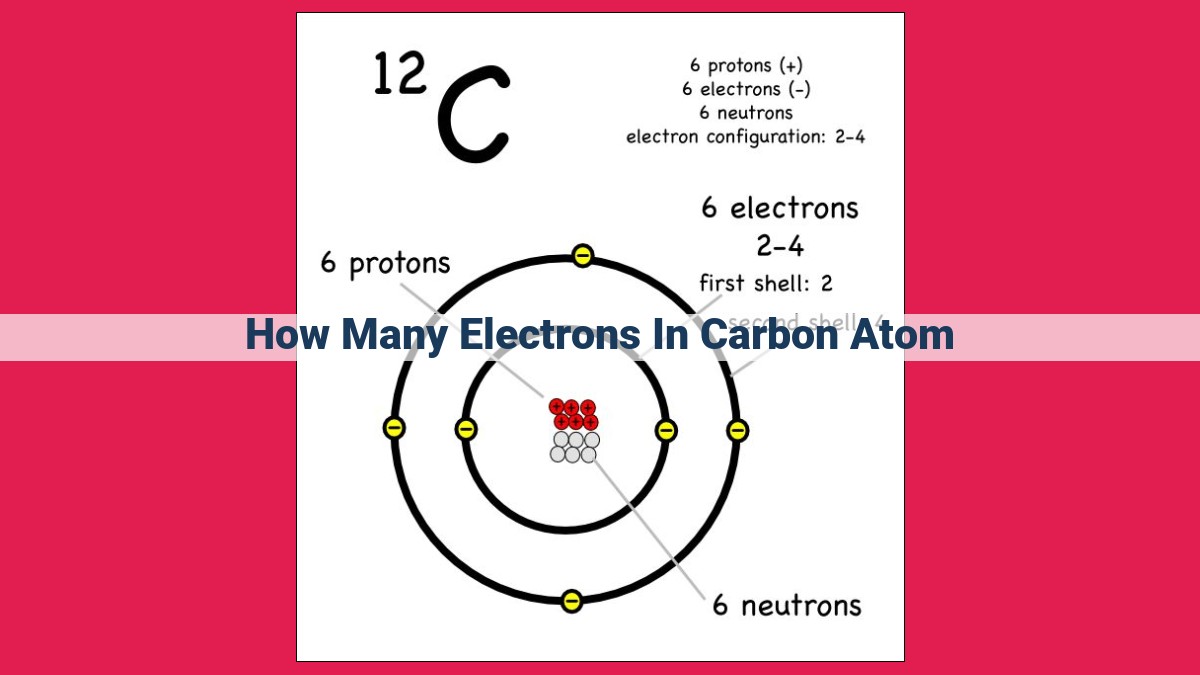
Carbon atoms have six electrons distributed across three energy levels. The atomic number of carbon (6) determines the number of protons and electrons. Electrons play a crucial role in chemical reactions, affecting an atom’s reactivity and bonding capabilities. Carbon’s four valence electrons (2s² 2p²) allow it to form bonds with various elements, making it a versatile and essential element in organic chemistry and life.
The Significance of Electrons: Unveiling the Building Blocks of Matter
In the realm of chemistry, electrons hold a pivotal position, shaping the very nature of the world around us. They are the fundamental components of atoms, the building blocks of all matter. Understanding the significance of electrons is crucial for deciphering the complexities of chemical reactions and unlocking the doors to countless scientific advancements.
An atom, the smallest unit of an element that retains its chemical properties, consists of a nucleus, a dense core containing protons (positively charged particles) and neutrons (neutral particles), and a surrounding cloud of electrons. Negatively charged and incredibly lightweight, electrons orbit the nucleus in specific energy levels, each level accommodating a set number of electrons.
Electrons play a key role in determining an atom’s chemical behavior. The number of electrons in an atom’s outermost energy level, known as valence electrons, determines its reactivity. Atoms with partially filled valence shells tend to be more chemically reactive, as they readily participate in bonding with other atoms to achieve a stable electron configuration.
Comprehending the significance of electrons empowers us to predict the behavior of atoms and molecules. This knowledge has profound implications in fields as diverse as chemistry, materials science, and biology. By deciphering the interplay between electrons and other atomic components, scientists can manipulate the properties of materials and design new compounds with tailored properties.
Further Reading:
Electrons: The Building Blocks of Matter
Understanding Electrons and Their Role in Chemistry
Understanding Atomic Number: The Defining Characteristic of Elements
When we delve into the world of atoms, their minuscule building blocks, we encounter a fundamental concept that shapes their identity: atomic number. Atomic number is the cornerstone of chemistry, defining the unique properties and characteristics of each element.
Imagine an atom as a tiny solar system, with a nucleus at its center and electrons orbiting around it. Protons, positively charged particles, reside in the nucleus, and their number determines an atom’s atomic number. This number is a unique fingerprint for each element.
The periodic table, a chart that organizes elements based on their atomic numbers, is a testament to this concept. Arranged in rows and columns, elements with similar atomic numbers share similar properties. For example, all elements in the same group (vertical column) have the same number of valence electrons, which are the electrons in their outermost energy level that determine their chemical behavior.
The atomic number of an element directly impacts its properties, such as its:
- Reactivity: Elements with low atomic numbers are generally more reactive, while those with high atomic numbers tend to be less reactive.
- Size: Atoms with low atomic numbers are usually larger than those with high atomic numbers, as the increased number of protons draws the electrons closer to the nucleus.
- Metallic vs. Nonmetallic Nature: Elements with low atomic numbers are more likely to be metals, while those with high atomic numbers are more likely to be nonmetals.
Understanding atomic number is crucial for comprehending the chemistry of elements. It unlocks the secrets of their behavior, allowing scientists to predict their reactivity, bonding preferences, and overall characteristics.
Section 2: Determining the Number of Electrons
Electrons: The Key Players in Chemistry
In the realm of chemistry, electrons take center stage as the fundamental building blocks of atoms. Understanding their number and distribution is crucial for unraveling the intricacies of chemical reactions.
Atomic Number: The Identity Card of Elements
Every element has a unique identity card, known as its atomic number. This number, denoted by the symbol Z, indicates the number of protons residing in the nucleus of an atom. Consequently, the number of electrons orbiting the nucleus is identical to the atomic number, ensuring electrical neutrality.
Valence Electrons: The Gatekeepers of Bonding
Among the electrons, a special group called valence electrons takes the spotlight. These electrons occupy the outermost energy level and have a strong influence on an atom’s chemical behavior. Their number determines the type and strength of bonds that an atom can form.
Electron Configuration: The Blueprint of Chemistry
The electron configuration of an atom reveals the specific arrangement of electrons in the energy levels around the nucleus. This blueprint provides valuable insights into the chemical properties of an element. For instance, atoms with similar electron configurations exhibit similar bonding patterns, leading to the organization of elements in the periodic table.
Section 3: Electron Configuration: The Arrangement of Electrons
Understanding the arrangement of electrons, known as electron configuration, is crucial to predicting the chemical behavior of any atom. It unveils the story of where electrons reside within an atom’s structure.
Imagine an atom’s nucleus, like a bustling city center, surrounded by different energy levels, akin to concentric circles. These circles, called atomic orbitals, are three-dimensional spaces where electrons reside. Each orbital can accommodate a maximum of two electrons, like two dancers on a stage.
Electron configuration describes how electrons are distributed across these energy levels starting from the lowest energy level. It’s like a seating chart for electrons, revealing their preferred energy levels. Electron configuration is usually represented using a symbolic notation that indicates the energy level and the number of electrons in each orbital.
The electron configuration holds the key to unlocking an atom’s chemical personality. It determines how atoms interact with each other, forming bonds and shaping the world around us. By deciphering the electron configuration of carbon, for instance, we can see why it forms the backbone of life, bonding effortlessly with various elements.
Carbon: The Versatile Element with Four Valence Electrons
In the realm of chemistry, electrons play a pivotal role, shaping atomic structures and dictating chemical reactions. Among the different elements, carbon stands out with its unique properties, owing largely to its electron configuration.
Understanding Carbon’s Atomic Number
Every atom has a specific atomic number, which represents the number of protons within its nucleus. Carbon’s atomic number is 6. This means that every carbon atom contains six protons, which define its position in the periodic table as element number 6.
Determining the Number of Electrons
In a neutral atom, the number of electrons equals the atomic number. Therefore, carbon atoms have six electrons. These electrons orbit the nucleus in specific energy levels, with the outermost level being the most reactive.
Valence Electrons: Carbon’s Chemical Keystone
The electrons in the outermost energy level are known as valence electrons. **Carbon has four valence electrons*, making it a highly reactive element. Valence electrons are responsible for forming chemical bonds, enabling carbon to combine with a vast array of other elements to create countless compounds.
Electron Configuration: Mapping Carbon’s Electrons
Electron configuration describes the arrangement of electrons within an atom. Carbon’s electron configuration is given as 1s² 2s² 2p². This notation indicates that two electrons occupy the 1s orbital, two in the 2s orbital, and two in the 2p orbital.
Importance of Valence Electrons in Carbon’s Bonding Capabilities
Carbon’s four valence electrons grant it the ability to form covalent bonds with other elements, sharing electron pairs to achieve a stable configuration. This versatility enables carbon to form a wide range of compounds, including organic molecules that are essential for life. Carbon’s bonding capabilities have revolutionized fields such as chemistry, materials science, and biology.