Carbon: The Foundation Of Organic Molecules And The Building Blocks Of Life
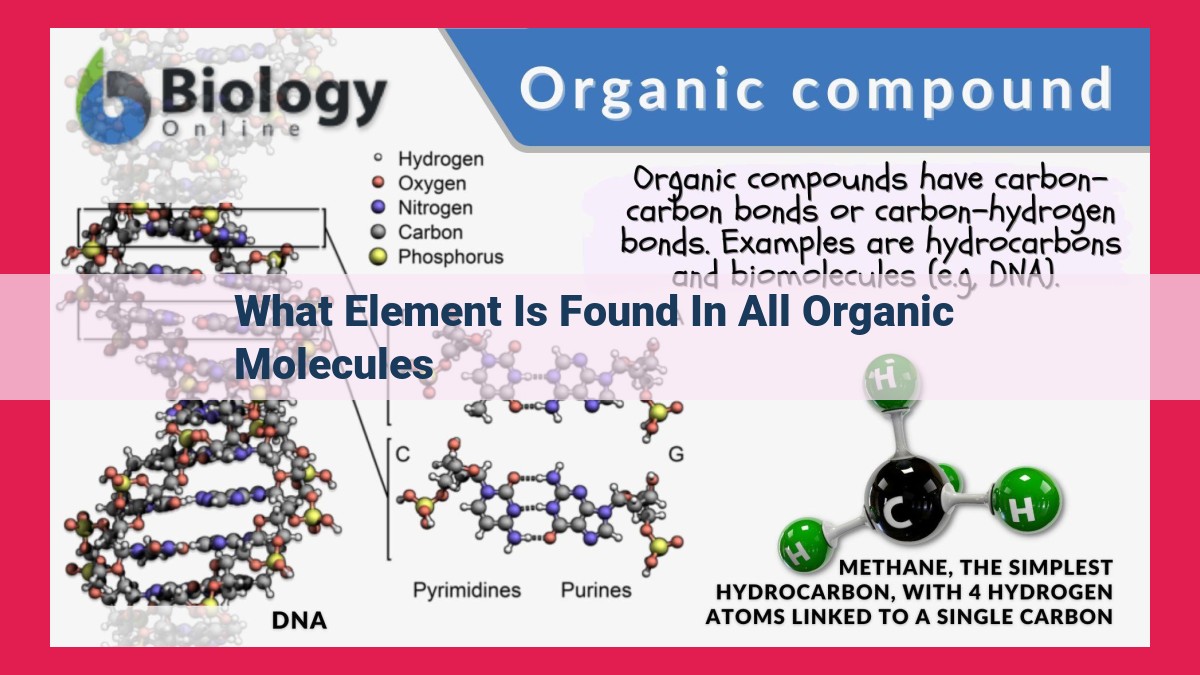
What Element is Found in All Organic Molecules?
Carbon is the backbone of all organic molecules, the building blocks of life. Its unique ability to form diverse bonds with itself and other elements allows for the creation of an endless array of organic compounds, including proteins, carbohydrates, lipids, and nucleic acids. These molecules are essential for all living organisms, enabling essential biological functions such as metabolism, energy production, and genetic inheritance.
Carbon: The Foundation of Life
Introduction:
Carbon, an extraordinary element, forms the very essence of life as we know it. It’s the building block of all organic molecules, the fundamental components of living organisms. From the tiniest microbes to the towering sequoias, carbon weaves its way through the intricate tapestry of life.
Ubiquitous Nature of Carbon:
The prevalence of carbon is truly remarkable. It’s the fourth most abundant element in the universe and the second most abundant in the human body. Its versatility is astonishing, capable of bonding with itself and a wide range of other elements to form countless compounds.
Organic Molecules: A Diverse Cosmos:
Organic molecules are the cornerstone of life. They include carbohydrates, proteins, lipids, and nucleic acids, each with its unique role. Carbohydrates provide energy, proteins form the building blocks of cells, lipids create membranes, and nucleic acids carry genetic information.
The Importance of Carbon in Organic Molecules:
Carbon’s unique properties enable it to form stable and complex structures. Its four valence electrons allow it to form covalent bonds with other carbon atoms and a variety of other elements, including hydrogen, oxygen, and nitrogen. This versatility gives rise to the vast array of organic molecules that make up living organisms.
Conclusion:
Carbon, the indispensable element, serves as the foundation of all life on Earth. Its ubiquitous presence, ability to form intricate structures, and role as the building block of organic molecules make it a crucial component in the symphony of life.
Organic Compounds: The Tapestry of Life
In the realm of chemistry, there exists a vast assembly of substances known as organic compounds. These intricate molecules, the building blocks of life, are ubiquitous in the natural world, from the intricate structures of DNA to the sweet nectar of flowers.
A Symphony of Variety
The universe of organic compounds is a kaleidoscope of diversity. Each molecule, a unique melody in this symphony, possesses a specific arrangement of carbon atoms. This versatile element serves as the backbone for these compounds, forming intricate scaffolds that can support an astonishing range of functional groups. These functional groups, like keys on a piano, endow each molecule with its own set of chemical properties and behaviors.
Essential to Life’s Processes
Organic compounds are indispensable to every facet of life. They form the fundamental scaffolding for biological molecules, such as carbohydrates, proteins, and lipids. These macromolecules orchestrate a dazzling array of cellular functions, from energy production to genetic information storage. Without the intricate tapestry of organic compounds, the symphony of life would cease to exist.
Exploring the Vastness
The vastness of the organic universe is truly awe-inspiring. It encompasses a myriad of classes, each with its own unique set of characteristics. Hydrocarbons, the simplest of organic molecules, serve as the building blocks for more complex compounds. Alcohols contain the hydroxyl functional group (-OH), lending them a range of properties that make them useful as solvents and fuels. Carboxylic acids possess an acidic functional group (-COOH), giving them the ability to donate protons in chemical reactions. These are but a few examples of the immense diversity found within the realm of organic compounds.
Inorganic Compounds: A Distinct Domain of Chemistry
In the realm of chemistry, molecules are divided into two broad categories: organic and inorganic. While we’ve delved into the world of organic compounds, it’s time to shift our focus to their counterpart: inorganic compounds. These substances, unlike their organic counterparts, lack the presence of carbon. This fundamental difference gives rise to distinct chemical properties that set them apart.
Inorganic compounds encompass a vast array of substances, including metals, salts, rocks, and minerals. They’re the building blocks of the inorganic world, forming the basis of our planet and many of the materials we use every day. Unlike organic compounds, which are based on a carbon backbone, inorganic compounds are characterized by their diverse compositions, often involving elements such as oxygen, hydrogen, nitrogen, and various metals.
The absence of carbon in inorganic compounds grants them a unique set of properties. They tend to be more stable and less reactive than organic compounds, making them suitable for various industrial and scientific applications. For instance, inorganic salts are essential for maintaining electrolyte balance in our bodies, while inorganic acids and bases play crucial roles in chemical reactions.
The study of inorganic compounds is a vast and complex field. It involves understanding their structures, properties, and reactivity. By delving into the intricate world of inorganic chemistry, scientists have developed a wealth of knowledge that has led to groundbreaking technologies and advancements in materials science, medicine, and beyond.
So, as we continue our exploration of the chemical world, let’s not forget the fascinating and diverse realm of inorganic compounds. These substances, despite their lack of carbon, play a vital role in shaping our world and enabling countless innovations that make our lives better.
Hydrocarbons: The Simplest Yet Versatile Organic Molecules
What are Hydrocarbons?
In the realm of organic chemistry, where life’s building blocks reside, hydrocarbons stand as the most basic and ubiquitous of all molecules. Composed solely of carbon and hydrogen atoms, these unassuming substances form the foundation upon which the vast tapestry of organic compounds is woven.
The Essence of Simplicity
Hydrocarbons are notable for their lack of complexity. They lack the functional groups that bestow specific chemical properties upon other organic molecules. This apparent simplicity, however, belies a surprising versatility. Hydrocarbons serve as the raw material for a myriad of more intricate compounds, including fuels, plastics, and pharmaceuticals.
The Role of Carbon and Hydrogen
Carbon atoms, with their ability to form strong covalent bonds with other carbon atoms and with hydrogen, are the backbone of hydrocarbons. These bonds create a stable, three-dimensional framework that can withstand the rigors of biological processes and chemical reactions. Hydrogen atoms, on the other hand, contribute stability and shape to the hydrocarbon molecule.
A Building Block for Complexity
Hydrocarbons are not mere simpletons, but rather essential building blocks for more complex organic molecules. They provide the skeletal framework upon which functional groups can be attached, giving rise to the vast array of compounds that are fundamental to life. Without hydrocarbons, the intricate machinery of cells, tissues, and organs would simply not exist.
Hydrocarbons, despite their apparent simplicity, play a pivotal role in the symphony of life. Their versatility and ability to serve as building blocks for more complex molecules make them indispensable to the biological world. From the fuels that power our vehicles to the plastics that shape our surroundings, hydrocarbons are truly the unsung heroes of organic chemistry.
Functional Groups: Unveiling the Chemical Diversity of Organic Molecules
Prepare to explore the fascinating world of functional groups, the molecular building blocks that unlock the astonishing chemical diversity of organic compounds. These unique atomic arrangements are the key to understanding the reactivity, behavior, and endless applications of these essential molecules.
Imagine a vast chemical library filled with countless organic molecules, each possessing a distinct personality shaped by its functional groups. These groups are like tiny chemical magnets, attracting specific types of reactions and interactions. They dictate whether a molecule is hydrophilic (water-loving) or hydrophobic (water-fearing), whether it can donate or accept electrons, and whether it can form bonds with other molecules.
The presence of functional groups can drastically alter a molecule’s properties. For instance, hydroxyl groups (-OH) give molecules the ability to form hydrogen bonds, making them polar and soluble in water. Carbonyl groups (C=O) impart reactivity and are essential for many biological processes. Amine groups (-NH2) confer basicity and can act as proton acceptors.
By tailoring the functional groups present in a molecule, scientists can design molecules with specific properties for a wide range of applications. From pharmaceuticals to plastics, from detergents to fragrances, the versatility of functional groups enables the creation of countless materials that enhance our lives.
Unveiling the Symphony of Chemistry
Functional groups are the conductors of the chemical orchestra, orchestrating the interactions between molecules and shaping the symphony of life. They determine the reactivity, behavior, and fate of organic molecules, providing the foundation for the intricate tapestry of chemical processes that occur within and around us.
By understanding the role of functional groups, we can not only comprehend the chemical world but also harness its power to create molecules that serve our needs and improve our lives.