Carbon: The Foundation Of Organic Molecules And Life On Earth
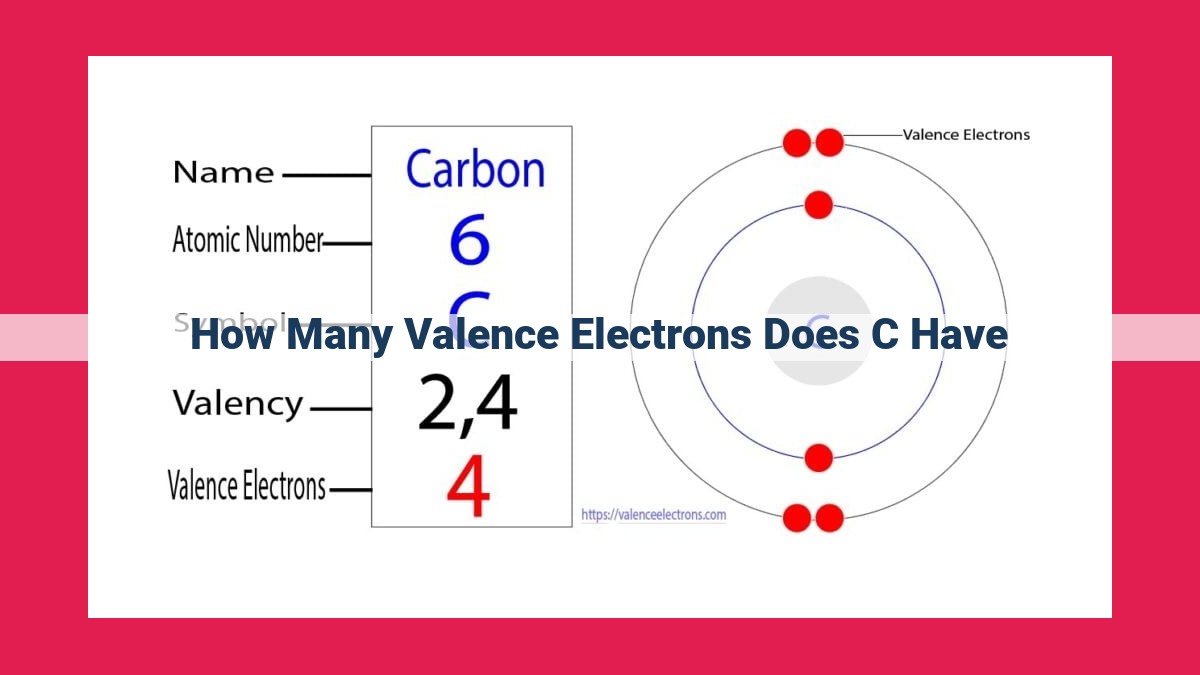
Carbon, the fundamental element of organic molecules, possesses six valence electrons in its outermost energy level. These electrons play a crucial role in carbon’s chemical versatility, enabling it to form covalent bonds with various other elements, including hydrogen, oxygen, and nitrogen. Carbon’s ability to form a diverse range of compounds underlies its significance in biological systems, contributing to the complexity and functionality of life on Earth.
How Many Valence Electrons Does Carbon Have?
Embark on a journey into the fascinating world of chemistry, where we unravel the secrets of carbon and its pivotal role in shaping our universe. In this blog, we will delve into the concept of valence electrons and discover the remarkable properties that make carbon the backbone of life and innovation.
What Are Valence Electrons?
At the heart of every atom lie electrons, orbiting the nucleus like tiny planets around a sun. Valence electrons are the electrons occupying the outermost energy level of an atom. These electrons play a crucial role in determining an atom’s chemical behavior and ability to form bonds with other atoms.
The number of valence electrons an atom possesses influences its reactivity, the tendency to engage in chemical reactions. Atoms with more valence electrons are generally more reactive than those with fewer.
Carbon: The Versatile Building Block
Carbon, with its atomic number 6, ranks among the most versatile elements on the periodic table. It has six valence electrons, making it a prime candidate for forming bonds and creating a vast array of molecules.
Carbon’s Chemical Versatility
The six valence electrons of carbon empower it with unparalleled bonding capabilities. These electrons can form covalent bonds, sharing electrons with other atoms, leading to the creation of countless organic molecules and biological systems. Carbon’s centrality in life processes stems from its ability to form long chains and complex structures that are essential for living organisms.
Connecting Electron Configuration to Valence Electrons
The number of valence electrons in an atom is directly correlated to its electron configuration, the arrangement of electrons in energy levels. For carbon, its electron configuration is 1s²2s²2p², with the six valence electrons residing in the 2p subshell. Understanding electron configurations helps us comprehend the chemical properties of elements and their ability to form bonds.
Significance in Carbon Chemistry
Grasping the concept of valence electrons is paramount for understanding the chemical properties of carbon and its extensive applications. Knowledge of valence electrons enables scientists to predict and design new materials, unravel biological processes, and develop medical advancements.
Valence electrons serve as the bridge between the atomic structure of carbon and its exceptional chemical versatility. Understanding the role of valence electrons has paved the way for groundbreaking discoveries and continues to drive innovation in various fields. From biology to materials science, the significance of valence electrons in carbon chemistry is undeniable.
How Many Valence Electrons Does Carbon Have?
1. What Are Valence Electrons?
Valence electrons, the stars of chemical reactions, reside in the outermost shell of an atom. These eager electrons determine an element’s bonding behavior, dictating its chemistry.
2. Carbon: The Versatile Building Block
Meet carbon, the central player in the world of chemistry. With an atomic number of 6, its electron configuration reveals its six valence electrons. These electrons, eager to connect, make carbon the master of bonding.
3. Carbon’s Chemical Versatility
Carbon’s valence electrons unlock a world of chemical possibilities. It can bond with itself and other elements, forming countless organic molecules. These molecules are the cornerstones of life on Earth.
Role in Forming Chemical Bonds
Valence electrons play a crucial role in forming chemical bonds, the bridges that connect atoms together. These bonds occur when valence electrons are shared or exchanged between atoms.
- Covalent Bonds: Carbon’s valence electrons share with other atoms, forming strong covalent bonds.
- Ionic Bonds: In ionic bonds, carbon’s valence electrons are transferred to other atoms, creating charged particles.
4. Connecting Electron Configuration to Valence Electrons
An atom’s electron configuration reveals the number of valence electrons. The number of electrons in the outermost shell corresponds to the number of valence electrons.
5. Significance in Carbon Chemistry
Understanding carbon’s valence electrons is paramount in unraveling its chemical properties. It allows scientists to predict reaction outcomes and develop novel materials.
Valence electrons are the key to understanding carbon’s chemistry. They unlock its versatility, enabling it to form countless molecules and participate in life’s processes. This knowledge empowers scientists to innovate and shape the future.
How Many Valence Electrons Does Carbon Have?
In the vast realm of chemistry, few elements play a role as pivotal as carbon. It weaves through the fabric of life, forms the backbone of our world, and holds the key to countless innovations. At the heart of carbon’s versatility lies a fundamental property: its valence electrons.
1. What Are Valence Electrons?
Valence electrons are the electrons that occupy the outermost energy level of an atom. They are the key players in chemical bonding, the process that connects atoms together to form molecules. These “social electrons” have a profound impact on an element’s chemical behavior.
2. Carbon: The Versatile Building Block
Carbon, with its atomic number 6, has a unique electron configuration: 1s²2s²2p². This means it has six valence electrons (two in the 2s orbital and four in the 2p orbital). This sextet of valence electrons gives carbon an uncanny ability to bond with a wide array of other atoms, creating an extraordinary diversity of molecules.
3. Carbon’s Chemical Versatility
Carbon’s six valence electrons endow it with exceptional bonding capabilities. It can form single, double, and triple bonds with other atoms, as well as participate in complex ring structures. This flexibility allows carbon to create an almost infinite array of organic molecules, the building blocks of life. From carbohydrates to proteins, DNA to pharmaceuticals, carbon is the “master molecule builder” behind every living organism.
4. Connecting Electron Configuration to Valence Electrons
The number of valence electrons in an atom is directly related to its electron configuration. The electron configuration describes the arrangement of electrons in an atom’s energy levels. The outermost energy level, filled with valence electrons, determines the atom’s chemical properties.
5. Significance in Carbon Chemistry
Understanding carbon’s valence electrons is fundamental to comprehending its chemical behavior. By knowing the number and arrangement of its valence electrons, scientists can predict how carbon will react with other elements and design innovative materials with tailored properties. This knowledge has applications in fields ranging from biology to materials science and medicine.
Valence electrons are the key to unlocking the mysteries of carbon’s extraordinary chemistry. Their role in chemical bonding and molecular formation makes carbon the cornerstone of life and the foundation for countless technological advancements. By understanding the importance of valence electrons, we empower ourselves to harness the power of carbon for the betterment of humanity.
How Many Valence Electrons Does Carbon Have?
In the enchanting realm of chemistry, carbon stands as an enigmatic yet crucial element, renowned for its versatility and life-giving properties. Unraveling the secrets behind carbon’s extraordinary nature begins with understanding its valence electrons. These electrons, like mischievous pixies, play a pivotal role in shaping carbon’s chemical destiny.
Carbon, with an atomic number of 6, boasts a peculiar electron configuration of 2, 4. This means that six electrons reside in carbon’s outermost energy level, eagerly awaiting the opportunity to form chemical bonds. These six valence electrons are the key to understanding carbon’s ability to interact with other elements and create countless molecules that shape our world.
Carbon’s six valence electrons赋予它非凡的化学能力。它们就像顽皮的小妖精,四处游走,与其他元素的原子牵手共舞,形成牢固的化学键。这种键合能力赋予了碳创造有机分子和生物系统的魔力,而这些分子和系统构成了生命的基础。
The intricate relationship between carbon’s electron configuration and valence electrons is akin to a delicate dance. The number of valence electrons directly corresponds to the atomic number of an element. By understanding this connection, chemists can predict the chemical behavior of elements and unravel the mysteries of their interactions.
Comprehending the significance of valence electrons in carbon chemistry is not merely an academic pursuit. It has profound implications for fields ranging from biology to materials science and medicine. By harnessing the power of carbon’s valence electrons, scientists can design new materials, develop innovative pharmaceuticals, and deepen our understanding of the intricate workings of life itself.
So, the next time you marvel at the complexities of life, remember the unsung heroes behind the scenes – carbon’s six valence electrons. They are the tiny architects that orchestrate the symphony of chemical reactions, shaping our world in ways we are only beginning to comprehend.
How Many Valence Electrons Does Carbon Have?
The Building Blocks of Life
In the vast tapestry of elements, carbon stands out as the universal building block of life. Its unique properties, fueled by the valence electrons it possesses, have shaped the very foundations of our existence. But what exactly are valence electrons, and how do they empower carbon with its remarkable versatility?
Valence Electrons: The Key to Bonding
Valence electrons are the outermost electrons in an atom, those that dance freely around the atomic nucleus. It’s these electrons that hold the secret to an atom’s chemical behavior. They determine an atom’s ability to form bonds with other atoms, the dance of electrons that gives rise to the molecules that make up our world.
Carbon’s Stellar Six
Carbon, with its atomic number of 6, boasts a remarkable six valence electrons. These six electrons occupy the outermost energy level of the carbon atom, like a lively entourage attending a cosmic ball. This electron sextet gives carbon an extraordinary ability to bond with a wide range of elements, forming the foundation of an astonishing array of molecules.
Carbon’s Bonding Magic
With its six valence electrons, carbon can form covalent bonds with up to four other atoms, creating a tetrahedral shape that resembles a tiny three-dimensional pyramid. These covalent bonds, the sharing of electrons between atoms, are the secret to carbon’s versatility. They allow carbon to connect with a dizzying array of elements, including itself, forming chains, rings, and complex three-dimensional structures.
The Carbon Superstar
Carbon’s bonding prowess has made it the superstar of chemistry, the essential component of countless organic molecules, the very molecules that form the basis of life. From the proteins that sustain us to the DNA that defines our genetic makeup, carbon is the indispensable player. Its ability to form covalent bonds with hydrogen, oxygen, nitrogen, and other elements has given rise to the immense diversity of life forms on Earth and beyond.
The Importance of Valence Electrons
Understanding the role of valence electrons in carbon chemistry is crucial for comprehending the chemical properties of this remarkable element. It provides insights into the formation and behavior of organic molecules, unlocking the secrets of biological systems and paving the way for advancements in fields such as medicine, materials science, and beyond.
How Many Valence Electrons Does Carbon Have?
Carbon is a crucial element that forms the foundation of life as we know it. Understanding its chemical properties is essential for unraveling the mysteries of biological processes and material sciences alike. At the heart of carbon’s versatility lies the concept of valence electrons.
In this blog post, we delve into the fascinating world of valence electrons, exploring their role in shaping carbon’s remarkable abilities. We’ll discover how these fundamental particles contribute to carbon’s unique bonding characteristics, the formation of organic molecules and biological systems, and its significance in countless fields of scientific inquiry. Join us as we unravel the secrets of carbon, one valence electron at a time!
Carbon: The Versatile Building Block
Carbon is an extraordinary element with an atomic number of 6. Its electron configuration—the arrangement of electrons around its nucleus—reveals that carbon has six electrons in its outermost energy level. These six electrons are known as valence electrons, and they play a pivotal role in determining carbon’s chemical properties.
Valence Electrons: The Key to Bonding
Valence electrons are the electrons in an atom’s outermost energy level. They are actively involved in forming chemical bonds with other atoms, a process that allows atoms to combine and form molecules. Atoms can gain, lose, or share valence electrons to achieve a stable electron configuration.
Carbon’s Bonding Capabilities
With its six valence electrons, carbon has exceptional bonding capabilities. It can form covalent bonds, sharing valence electrons with other atoms to create stable molecular structures. This ability is the cornerstone of carbon’s unparalleled versatility in forming a vast array of compounds.
Formation of Organic Molecules and Biological Systems
Carbon’s valence electrons play a central role in the formation of organic molecules—the building blocks of life. Organic molecules contain carbon atoms bonded to other elements such as hydrogen, oxygen, and nitrogen. The diversity of organic molecules arises from the ability of carbon atoms to form chains, rings, and complex three-dimensional structures.
Carbon’s Centrality in Life Processes
The abundance of organic molecules formed by carbon makes it the central element in all known biological systems. From the smallest bacteria to the largest whales, the intricate structures and functions of living organisms rely heavily on carbon’s remarkable bonding properties. Carbon-based molecules, such as DNA and proteins, carry genetic information, facilitate chemical reactions, and provide structural support.
Significance in Carbon Chemistry
Understanding the behavior of carbon’s valence electrons is crucial for comprehending the chemical properties of carbon-based compounds. By unraveling the intricacies of carbon’s electron configuration, scientists can predict the reactivity, stability, and functionality of these compounds. This knowledge underpins countless applications in fields such as biology, materials science, and medicine.
How Many Valence Electrons Does Carbon Have?
Carbon, an extraordinary element, holds the key to life on Earth. Its unique properties stem from its atomic structure, particularly the number of valence electrons it possesses.
Carbon’s Centrality in Life Processes
Carbon’s six valence electrons play a pivotal role in its ability to form diverse compounds, making it indispensable for biological processes. These bonds with other atoms, such as hydrogen, oxygen, and nitrogen, give rise to the complex molecules that form the basis of life.
From the proteins and nucleic acids in our cells to the carbohydrates that provide energy, carbon is the fundamental building block of all living organisms. Its versatility in forming bonds allows it to create an array of molecules with varying shapes and functions.
Examples of Carbon’s Role in Life
- Enzymes: Carbon-containing enzymes catalyze biochemical reactions, facilitating essential processes like digestion and metabolism.
- Photosynthesis: Carbon dioxide is the raw material for photosynthesis, the process by which plants convert sunlight into energy.
- Respiration: Carbon dioxide is released as a byproduct of cellular respiration, supplying energy for life.
Carbon-Based Technology and Applications
The understanding of carbon’s valence electrons has revolutionized science and technology. Carbon-based materials, such as graphene and carbon nanotubes, possess remarkable properties that make them ideal for applications in:
- Electronics: High electrical conductivity and thermal stability.
- Materials science: Exceptional strength and lightweight construction.
- Medicine: Drug delivery and tissue engineering.
Carbon’s six valence electrons are the cornerstone of its chemical versatility. They not only make it a central element in life processes but also enable the development of cutting-edge technologies. By unraveling the secrets of carbon’s atomic structure, we continue to unlock its vast potential and shape the future of science, medicine, and beyond.
How Many Valence Electrons Does Carbon Have?
What Are Valence Electrons?
Valence electrons are the electrons in an atom’s outermost shell, which determine its chemical behavior. They are crucial for forming chemical bonds, the glue that holds atoms together to create molecules.
Carbon: The Versatile Building Block
Carbon is the backbone of life, found in everything from our DNA to the graphite in our pencils. Its unique properties stem from its six valence electrons. With six electrons in its outermost shell, carbon can bond with almost any other element, making it the foundation of countless molecules.
Carbon’s Chemical Versatility
Carbon’s valence electrons enable it to form single, double, and even triple bonds, creating an array of organic molecules, which are the basis of all living organisms. This versatility also allows carbon to play a central role in materials science and other fields.
4. Connecting Electron Configuration to Valence Electrons
The relationship between an atom’s structure and its valence electrons is crucial. Electrons are arranged in shells, with valence electrons occupying the outermost shell. The number of valence electrons directly influences the atom’s chemical properties.
Significance in Carbon Chemistry
Understanding carbon’s valence electrons is essential for comprehending its chemical behavior. This knowledge underpins the development of new materials, medications, and technologies in fields such as biology, materials science, and engineering.
Valence electrons are the key to understanding carbon’s chemistry and its wide-ranging applications. By grasping the role of valence electrons, we can unlock the secrets of this versatile element and harness its potential in shaping our world.
How Many Valence Electrons Does Carbon Have?
Carbon, the foundation of all life on Earth, is an element with a remarkable ability to form diverse chemical bonds. This versatility stems from its unique valence electrons, the electrons in its outermost shell that determine its chemical behavior.
Valence Electrons and Chemical Bonding
Valence electrons are the electrons that participate in chemical reactions. They determine an element’s ability to form bonds with other atoms. Carbon has six valence electrons, which gives it the potential to form multiple bonds.
The Structure of Carbon
Carbon’s atomic number is 6, meaning it has six electrons. The electron configuration of carbon is 1s²2s²2p², indicating that it has two electrons in the 1s orbital, two in the 2s orbital, and two in the 2p orbital. The 2p orbital contains three degenerate orbitals (2px, 2py, 2pz), each of which can hold two electrons.
Concept of Atomic Orbitals and Electron Pairing
Atomic orbitals are regions around the nucleus where electrons are most likely to be found. The three 2p orbitals form a dumbbell-shaped structure. The two electrons in the 2p orbitals pair up, occupying the same orbital with opposite spins.
Carbon’s Chemical Versatility
Carbon’s six valence electrons allow it to form bonds with various elements, including hydrogen, oxygen, nitrogen, and itself. This versatility makes carbon the backbone of organic molecules, the building blocks of life. Carbon can form single, double, or triple bonds, enabling it to create complex and diverse structures.
Significance in Carbon Chemistry
Understanding the number of valence electrons is crucial for predicting the chemical properties of carbon and its compounds. This knowledge has applications in fields such as:
- Biology: Carbon’s ability to form diverse bonds is essential for the structure and function of biological molecules.
- Materials science: Carbon-based materials, such as graphene and nanotubes, have remarkable properties and potential applications.
- Medicine: Carbon-containing compounds are used in drugs, diagnostics, and medical devices.
The six valence electrons of carbon are fundamental to its extraordinary versatility and central role in chemistry. Understanding this concept is essential for unraveling the mysteries of carbon chemistry and its wide-ranging applications in various scientific disciplines.
Understanding the chemical properties of carbon based on its valence electrons
How Many Valence Electrons Does Carbon Have?
Carbon, the sixth element on the periodic table, holds a remarkable place in our universe, playing a central role in the chemistry of life. To unlock the secrets behind carbon’s versatility, we must dive into the realm of valence electrons.
What Are Valence Electrons?
Imagine electrons dancing around the nucleus of an atom, like energetic children at a party. Valence electrons are those located in the outermost shell of this atomic dance, responsible for forming chemical bonds with their buddies from neighboring atoms.
Carbon: The Versatile Building Block
Carbon’s atomic number is six, indicating that its nucleus houses six protons. Its electron configuration is 1s²2s²2p², revealing six electrons dancing in its outermost shell. These six valence electrons grant carbon an incredible chemical versatility.
Exploring Carbon’s Chemical Prowess
Carbon’s valence electrons enable it to form a vast array of chemical bonds. It can bond with itself, forming long chains and rings of carbon atoms. These bonds give rise to the extraordinary diversity of organic molecules, the building blocks of life.
Even more intriguing, carbon’s versatility extends to bonding with other elements, such as hydrogen, oxygen, and nitrogen. This flexibility allows it to create complex biological systems, from proteins to DNA, the blueprints of life’s intricate tapestry.
Electron Configuration: The Key to Understanding
The number of valence electrons is inextricably linked to an atom’s electron configuration. The arrangement of electrons in orbitals, the energy levels surrounding the nucleus, determines which electrons are available for bonding.
Unveiling Carbon Chemistry’s Significance
Comprehending carbon’s valence electrons unlocks the door to understanding its chemical properties. This knowledge forms the foundation for advancements in biology, where carbon-based molecules govern life’s processes. It also fuels progress in materials science and medicine, where carbon’s unique properties hold immense potential.
In conclusion, the valence electrons of carbon are the key to unlocking its chemical versatility and understanding its profound impact on our world. These tiny particles dance within the heart of carbon atoms, guiding its ability to bond and create the intricate fabric of life and technology.
How Many Valence Electrons Does Carbon Have?
1. What Are Valence Electrons?
- Valence electrons are the electrons in the outermost energy level of an atom.
- They play a crucial role in chemical bonding by determining how an atom interacts with other atoms.
2. Carbon: The Versatile Building Block
- Carbon has atomic number 6 and an electron configuration of 1s2 2s2 2p2.
- It has six valence electrons, making it highly versatile in forming bonds.
3. Carbon’s Chemical Versatility
- Organic molecules: Carbon forms the backbone of organic molecules, which are essential for life.
- Biological systems: Carbon is the foundation of biological systems, including DNA and proteins.
- Life processes: Carbon’s unique properties make it central to numerous life processes, such as respiration and photosynthesis.
4. Connecting Electron Configuration to Valence Electrons
- The electron configuration of an element determines the number of valence electrons it has.
- Each energy level can hold a specific number of electrons, with the outermost level having the valence electrons.
5. Significance in Carbon Chemistry
- Understanding valence electrons is crucial for comprehending carbon’s chemical properties.
- It explains carbon’s ability to form diverse bonds and its role in organic and biological chemistry.
6. Applications in Fields Such as Biology, Materials Science, and Medicine
- Biology: The study of carbon chemistry helps scientists understand the structure and function of biological molecules.
- Materials science: Carbon-based materials, such as carbon nanotubes, have revolutionized the development of new materials with exceptional strength and conductivity.
- Medicine: Understanding carbon’s chemical properties aids in the development of drugs, medical imaging technologies, and tissue engineering advancements.
Valence electrons play a pivotal role in understanding the chemistry of carbon. Their unique properties have enabled carbon to become the foundation of life and a cornerstone in various scientific and technological fields. By delving into the fascinating world of valence electrons, we continue to unlock the potential of carbon, opening up new possibilities and innovations for the future.
Summarize the importance of valence electrons in understanding carbon’s chemistry
Summarize the Importance of Valence Electrons in Understanding Carbon’s Chemistry
The Keystone of Carbon’s Versatility
Carbon’s six valence electrons, residing in its outermost electron shell, play a crucial role in its remarkable chemical versatility. These electrons determine carbon’s bonding capabilities, enabling it to form covalent bonds with a vast array of elements, including itself. This electron-sharing ability is the cornerstone of carbon’s ability to create diverse and complex organic molecules.
The Foundation of Life and Beyond
Carbon’s valence electrons make it the building block of life. It is the central atom in organic compounds, the basis of DNA and proteins, and the very essence of living organisms. Its ability to form stable, interconnected structures allows for the intricate biomolecules that drive life’s processes.
Applications across Disciplines
Understanding carbon’s valence electrons has profound implications for numerous fields. In biology, it helps explain the formation and function of biological structures. In materials science, it guides the design of advanced materials, such as graphene and carbon nanotubes. In medicine, it aids in understanding drug interactions and developing new treatments.
The valence electrons of carbon stand as the keystone to its exceptional chemical versatility. Their significance extends far beyond understanding the behavior of this element; it permeates the very fabric of life and underpins countless applications across scientific disciplines. By embracing the power of carbon’s valence electrons, we unlock the potential to unravel new frontiers in science and technology, ultimately expanding our knowledge and improving the human experience.
Highlight the practical applications of this knowledge in various disciplines
How Many Valence Electrons Does Carbon Have?
Carbon, the foundation of all life on Earth, holds a captivating duality: its simplicity yet boundless versatility. To unravel its secrets, we must delve into the realm of valence electrons, the outermost electrons that determine its chemical behavior.
Carbon’s Six Valence Electrons
Carbon’s atomic number of six grants it exactly six valence electrons. These electrons reside in the outermost energy level, eagerly seeking out partners to form stable bonds. This eagerness drives carbon’s extraordinary chemical flexibility.
Carbon: The Chemical Chameleon
With its six valence electrons, carbon can bond with almost any other element, assuming a myriad of shapes and sizes. This bonding prowess enables carbon to create the vast diversity of organic molecules that underpin life. From the carbohydrates that fuel our bodies to the DNA that carries our genetic code, carbon’s versatility is a cornerstone of biological systems.
Connecting Electrons to Chemistry
Carbon’s unmatched chemical adaptability stems from the arrangement of its valence electrons in specific orbitals. These orbitals are like electron parking spaces, with the outermost occupied by the valence electrons. The configuration of these orbitals dictates carbon’s chemical identity, empowering it to form different types of bonds.
Practical Applications Across Disciplines
The profound importance of carbon’s valence electrons extends beyond biological processes. Its chemical prowess finds applications in a wide spectrum of fields, including:
- Biology: Designing new drugs, understanding cellular processes, and unraveling the mysteries of life itself.
- Materials Science: Creating stronger and lighter materials, such as carbon fibers used in aerospace and automotive engineering.
- Medicine: Developing treatments for cancer, heart disease, and other life-threatening conditions.
- Energy Storage: Harnessing carbon’s ability to store energy in batteries and fuel cells for sustainable energy solutions.
In conclusion, the significance of carbon’s valence electrons cannot be overstated. Their ability to form diverse bonds underpins the astonishing range of carbon-based compounds, from the building blocks of life to the cutting-edge technologies that shape our world. Understanding these valence electrons unlocks the gateway to unlocking the endless possibilities of carbon chemistry.