Unveiling Carbon’s Chemical Bonding Power: Atomic Structure And Electron Configuration
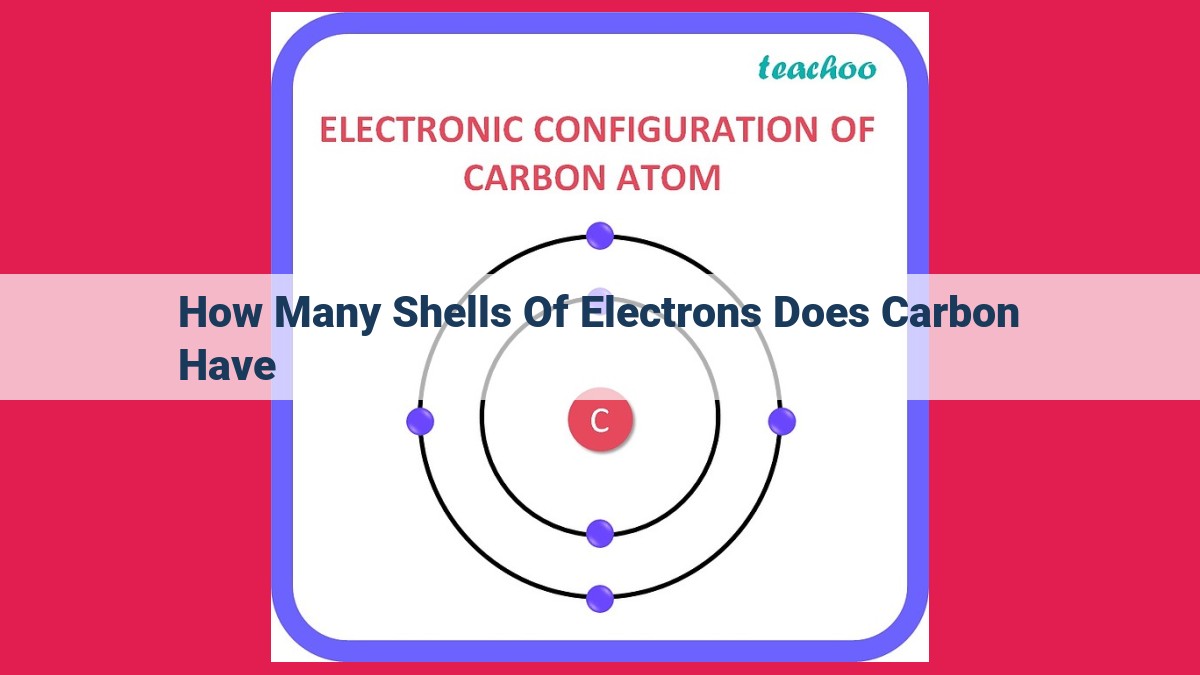
Carbon, the building block of life, has an atomic number of 6, indicating 6 electrons. Its electron configuration, 1s²2s²2p², reveals three shells. The first shell is filled (2 electrons), the second shell contains 2 electrons, and the third shell has 2 electrons. This three-shell structure is crucial for carbon’s chemical bonding capabilities, as it has four valence electrons (electrons in the outermost shell) available for bonding with other atoms.
Carbon: The Essential Element of Life
Carbon, the sixth element in the periodic table, holds a paramount position in the tapestry of life. It forms the backbone of all organic molecules, the building blocks of living organisms. Carbon’s unique properties distinguish it from its elemental counterparts, making it an indispensable component for the existence and sustenance of life on Earth.
The Significance of Carbon
Carbon’s significance lies in its unparalleled versatility and ability to form diverse covalent bonds. Its atomic number, 6, determines the number of electrons in its atom, 6, enabling it to form four covalent bonds. This tetravalence allows carbon to connect with other carbon atoms or various elements, creating a vast array of molecules with distinct shapes and functions.
Carbon’s Electron Configuration
The electron configuration of carbon, denoted as 1s² 2s² 2p², reveals the arrangement of its 6 electrons. The first two electrons occupy the 1s orbital, the second two reside in the 2s orbital, and the final two are found in the 2p orbital. This configuration plays a crucial role in carbon’s chemical bonding capabilities.
The Significance of Carbon’s Electron Shells
Carbon possesses three electron shells, indicated by the three numbers in its electron configuration. The outermost shell, containing two electrons, represents carbon’s valence electrons. These valence electrons determine the atom’s bonding behavior, enabling carbon to form single, double, or triple covalent bonds with other elements.
Understanding the number of electron shells in carbon is fundamental to comprehending its chemical properties and biological functions. This knowledge empowers us to unravel the intricate mechanisms underlying the vast array of organic molecules that govern life’s processes.
Carbon’s Atomic Structure: Unraveling the Building Blocks of Life
At the heart of every living organism lies an element so extraordinary that it’s often referred to as the “backbone of life.” That element is carbon, and its remarkable properties stem from its very structure.
Atomic Number and Electron Dance
Delving into carbon’s atomic structure, we encounter its defining characteristic: an atomic number of 6. This number signifies the presence of 6 protons in the atom’s nucleus, creating a positive charge. The number of protons also dictates the number of electrons that orbit the nucleus, maintaining electrical neutrality. Thus, carbon atoms have exactly 6 electrons, each contributing to the atom’s unique behavior.
Electrons in Motion: The Quantum Realm
These 6 electrons reside in shells or energy levels encircling the nucleus. Carbon’s electron configuration reveals a peculiar arrangement: 2 electrons occupy the innermost shell, while the remaining 4 electrons reside in the second shell. This specific configuration, 1s2 2s2 2p2, plays a pivotal role in carbon’s chemical reactivity and ability to form countless compounds.
The Number of Electrons in Carbon: A Key to Its Chemical Versatility
Carbon, the fundamental building block of life, possesses unique properties that make it indispensable in the intricate tapestry of living systems. At the heart of these remarkable attributes lies the number of electrons that dance around the carbon atom’s nucleus. This number, dictated by the atom’s atomic number, plays a pivotal role in determining carbon’s chemical behavior and, by extension, its ability to form the myriad molecules that underpin life.
Atomic Number and Electron Count
Each element in the periodic table is identified by its atomic number, which represents the number of protons within the atom’s nucleus. Carbon, occupying the sixth position in the table, boasts an atomic number of 6, indicating that its nucleus harbors six protons.
In a neutral atom, the number of electrons revolving around the nucleus exactly matches the number of protons. This delicate balance ensures that the atom maintains a neutral electrical charge. Thus, carbon possesses six electrons.
Importance in Chemical Bonding
The number of electrons in an atom profoundly influences its chemical reactivity, particularly its ability to form bonds with other atoms. When atoms interact, they aim to achieve a stable electron configuration, often by sharing or transferring electrons.
Carbon’s six electrons are strategically arranged in three electron shells. The outermost shell, known as the valence shell, contains four electrons. Valence electrons are the ones most actively involved in chemical bonding, as they can be shared, donated, or accepted to form stable bonds.
Carbon’s Chemical Versatility
The four valence electrons endow carbon with exceptional versatility, enabling it to form covalent bonds with itself and a vast array of other elements, including hydrogen, oxygen, nitrogen, and halogens. These bonds form the structural backbone of organic molecules, the building blocks of all living organisms.
Carbon’s ability to form covalent bonds with multiple atoms simultaneously gives rise to a staggering diversity of molecular structures, from the simple methane molecule (CH₄) to the complex and intricate biomolecules that orchestrate life’s processes.
The number of electrons in carbon, dictated by its atomic number, is a fundamental determinant of its chemical reactivity and versatility. With four valence electrons, carbon can engage in myriad bonding interactions, leading to the formation of an astonishing array of molecules that underpin the very fabric of life.
Electron Configuration of Carbon: Unraveling the Building Blocks of Life
Carbon, the cornerstone of life on our planet, owes its versatility to its unique electron configuration. Its atomic structure, with three electron shells, orchestrates its chemical behavior and biological significance.
The Language of Electron Configuration
Electron configuration, a symbolic shorthand, describes the arrangement of electrons within an atom’s shells. Each shell, designated by a number (n), can hold a specific number of electrons. For carbon, the electron configuration is written as 1s² 2s² 2p². This notation indicates:
- 1s²: Two electrons in the first shell (n = 1)
- 2s²: Two electrons in the second shell (n = 2)
- 2p²: Two electrons in the second shell (n = 2), in the p subshell
The Mystery of the Third Shell
Unlike the first two shells, which are fully filled with two electrons each, the third shell of carbon remains incomplete. It houses only four electrons instead of the possible six. This unfilled shell (2p⁴) holds the key to carbon’s remarkable chemical reactivity.
The valency of an element, the number of electrons available for bonding, is determined by the unfilled outermost shell. Carbon’s four valence electrons empower it to form covalent bonds with other atoms, creating the intricate networks that constitute the building blocks of our world.
SEO Optimized Keywords:
- Carbon electron configuration
- Electron shells in carbon
- Electron configuration notation
- Valence electrons in carbon
- Chemical behavior of carbon
- Biological significance of carbon
Number of Electron Shells in Carbon
Carbon, the fundamental building block of life, holds immense significance in the scientific realm. Understanding its atomic structure is crucial for unraveling its unique properties. After exploring carbon’s atomic number, the total number of electrons, and its electron configuration, we delve into the concept of electron shells.
Carbon’s electron configuration, 1s²2s²2p², reveals that it possesses three electron shells. Each shell can accommodate a specific number of electrons: two in the first shell, eight in the second, and eight in the outermost third shell.
The distribution of electrons among these shells influences carbon’s chemical behavior. The first shell, being closest to the nucleus, is highly stable with its two tightly bound electrons. The second shell, with another two electrons, is also relatively stable. However, the third shell plays a pivotal role in carbon’s chemistry.
It contains four electrons, leaving four valence electrons available for bonding. These valence electrons determine carbon’s ability to form covalent bonds with other atoms, enabling it to create a vast array of molecules and compounds.
Understanding the number of electron shells in carbon is fundamental to comprehending its chemical versatility and its role in countless biological processes. From the formation of organic molecules to the intricate structures of life, carbon’s unique electron configuration makes it an indispensable element in the symphony of life.
The Significance of Carbon’s Atomic Mass
Carbon: The Building Block of Life
Carbon, the sixth element in the periodic table, is the fundamental building block of all known life forms. Its unique properties, such as its ability to form diverse molecular structures, make it essential for countless organic compounds found in living organisms.
Atomic Mass: A Measure of an Atom’s Weight
Every element has a specific atomic mass, which represents the average mass of its atoms. Atomic mass is measured in atomic mass units (amu), with one amu being approximately equal to the mass of a single proton. The atomic mass of carbon is 12.011 amu.
Calculating Carbon’s Atomic Mass
The atomic mass of an element is calculated based on the abundance and masses of its isotopes. Isotopes are atoms of the same element that have the same number of protons but different numbers of neutrons. Carbon has three naturally occurring isotopes:
- Carbon-12: 98.89% abundance, 12 amu
- Carbon-13: 1.11% abundance, 13 amu
- Carbon-14: Trace amount, 14 amu
The atomic mass of carbon is determined by multiplying the abundance of each isotope by its mass and summing the results. This calculation yields an atomic mass of approximately 12.011 amu.
Importance of Carbon’s Atomic Mass
Understanding the atomic mass of carbon is crucial for:
- Determining the molecular weights of carbon-containing compounds
- Making calculations in chemistry involving carbon-based molecules
- Characterizing carbon-bearing materials, such as organic molecules and biological tissues
Atomic Radius: Unveiling the Size of Carbon Atoms
In the realm of chemistry, understanding the atomic structure of elements is crucial. One key concept is the atomic radius, which measures the distance from the nucleus to the outermost electron shell. For carbon, a fundamental building block of life, its atomic radius plays a critical role in determining its chemical properties and behavior.
Defining the Atomic Radius
The atomic radius is a measure of the size of an atom. It can be visualized as the distance between the nucleus, where protons and neutrons reside, and the outermost electron shell, where electrons orbit. The units of atomic radius are typically expressed in picometers (pm), one trillionth of a meter.
Carbon’s Atomic Radius
The atomic radius of carbon is approximately 70 pm. This value indicates that carbon atoms are relatively small compared to other elements. The small atomic radius of carbon contributes to its ability to form strong covalent bonds with other atoms, such as hydrogen, oxygen, and nitrogen.
Importance of Atomic Radius
The atomic radius of an element influences its chemical reactivity and physical properties. A smaller atomic radius generally means that the element is more reactive because its electrons are closer to the nucleus and more easily pulled away. In the case of carbon, its small atomic radius enhances its ability to participate in chemical reactions and form molecules.
Closing Thoughts
Understanding the atomic radius of carbon provides valuable insights into the behavior of this remarkable element. The small atomic radius of carbon explains its high reactivity and ability to bond with a wide range of other atoms. This knowledge is essential for comprehending carbon’s diverse chemistry, including its role as the backbone of organic molecules and the foundation of life as we know it.
Carbon’s Ionization Energy: Unlocking the Secret to Its Chemical Reactivity
Carbon, the building block of life, holds a fascinating position in the realm of chemistry due to its unique properties. One significant aspect that influences its reactivity is its ionization energy.
Understanding Ionization Energy:
Ionization energy refers to the energy required to remove an electron from an atom or ion. It is a crucial concept in chemistry as it provides insights into an element’s tendency to form chemical bonds. For carbon specifically, its ionization energy plays a pivotal role in shaping its chemical behavior.
Impact on Chemical Reactivity:
Low Ionization Energy: Carbon’s ionization energy is relatively low compared to other elements. This means that carbon can easily lose electrons, making it more likely to form bonds with other atoms that have a greater affinity for electrons. This property makes carbon an excellent electron donor, enabling it to participate in a wide range of chemical reactions.
Importance for Carbon’s Role in Life:
Carbon’s low ionization energy is essential for its role in biological systems. The ability to form stable covalent bonds allows carbon to create the complex molecules that are the foundation of life, such as proteins, carbohydrates, and nucleic acids.
Applications in Science and Technology:
Carbon’s low ionization energy has significant applications across various fields:
- Organic Chemistry: Carbon’s ability to form bonds with itself and other elements forms the basis of organic chemistry, the study of carbon-based molecules.
- Materials Science: Carbon’s ionization energy affects the properties of materials, such as graphene and carbon nanotubes, leading to advancements in electronics, energy storage, and material science.
- Nuclear Chemistry: Carbon-14, an isotope with a radioactive nucleus, is used in radiocarbon dating to determine the age of ancient artifacts and fossils.
The ionization energy of carbon is a fundamental property that governs its chemical reactivity. Its low value allows carbon to participate in numerous chemical reactions, making it essential for the existence of life and the development of various technologies. Understanding the significance of carbon’s ionization energy provides a deeper appreciation for its crucial role in chemistry and its impact on the world we live in.
Valence Electrons: Carbon’s Magic Ingredient for Bonding
In the realm of chemistry, carbon stands as a true elemental superstar. Its unique properties stem from its intricate atomic structure, particularly the number of electrons it possesses. One aspect that sets it apart is the presence of valence electrons, which play a crucial role in carbon’s ability to bond with other atoms.
Valence Electrons: The Key to Bonding
Picture a chemical bond as a molecular handshake between atoms. Valence electrons, located in the atom’s outermost shell, are the hands that reach out to form these bonds. In the case of carbon, it has four valence electrons, making it a bonding powerhouse.
Carbon’s Bonding Prowess
These four valence electrons allow carbon to form single, double, or even triple bonds with other atoms. This versatility is the secret behind the astonishing variety of carbon-based molecules that exist, from the simple carbon dioxide in the air we breathe to the complex DNA that carries our genetic code.
Understanding the number of electron shells in carbon is essential for comprehending its chemical behavior and biological functions. Its four valence electrons are the driving force behind its exceptional bonding capabilities, enabling the creation of an infinite array of molecules that make life on Earth possible.