Calculating Rate Constants: Theoretical Frameworks And Practical Applications
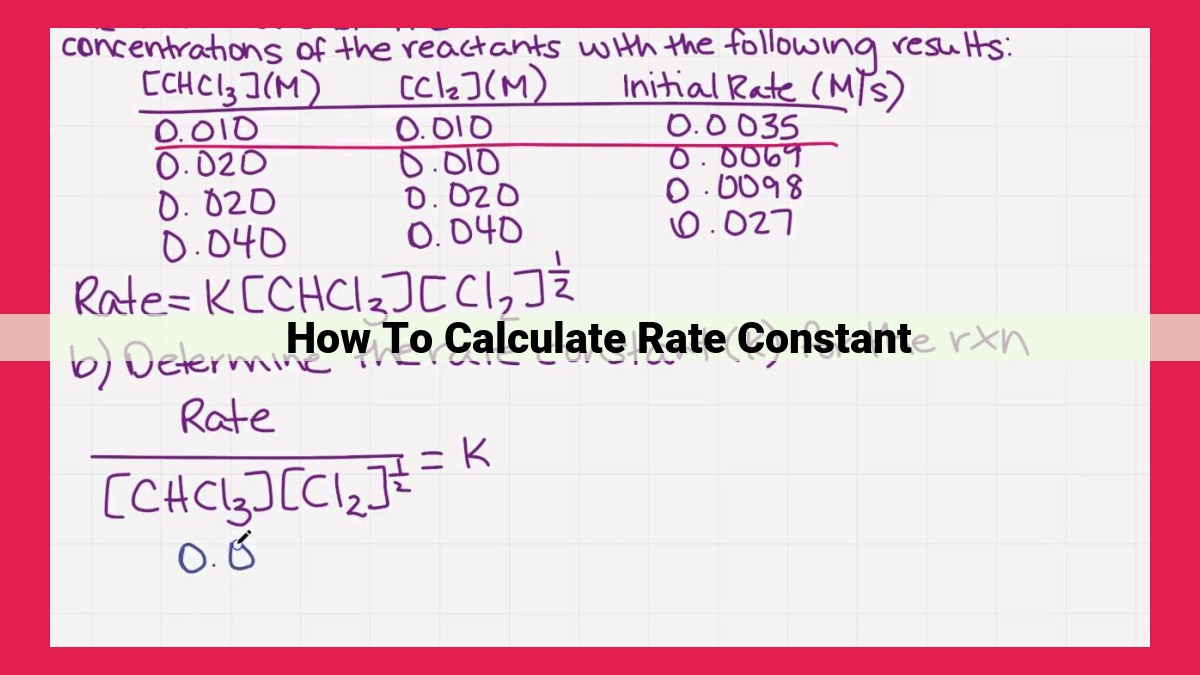
To calculate rate constants, various methods can be employed: Collision and Activated Complex Theory provide a framework for understanding reaction rates, with activated complex theory explaining the formation of a high-energy intermediate. The Arrhenius, Eyring, and Transition State Theory equations relate rate constants to temperature and activation energy. The free energy of activation signifies the energy barrier for reactions, while the rate-determining step identifies the slowest step in a reaction mechanism. Reaction mechanisms aid in deriving rate expressions, and understanding temperature and concentration dependence through Arrhenius and Eyring equations is crucial. By utilizing these concepts, rate constants can be accurately determined, enabling researchers to analyze reaction kinetics and predict reaction outcomes.
Collision and Activated Complex Theory
- Describe collision theory and its limitations.
- Explain activated complex theory and its importance.
Collision and Activated Complex Theory: Understanding Chemical Reactions
The world of chemistry is a fascinating realm where countless reactions occur at the molecular level. Chemical kinetics, the study of reaction rates, is crucial for comprehending these intricate processes. At the heart of chemical kinetics lies the concept of collision theory, which explains how reactions occur when reactant molecules collide with sufficient energy to overcome an activation energy barrier.
Collision Theory and Its Limitations
Collision theory postulates that chemical reactions occur when reactant molecules collide with each other. However, this theory alone cannot fully account for the observed reaction rates. According to collision theory, the rate of a reaction is directly proportional to the frequency of collisions between reactants. However, not all collisions are effective.
Activated Complex Theory and Its Importance
To address the limitations of collision theory, scientists developed the activated complex theory. This theory proposes that reactant molecules must first form an unstable intermediate species known as the activated complex. The activated complex is a high-energy state that exists only momentarily before transforming into product molecules. The activation energy is the minimum amount of energy that must be supplied to reactant molecules to reach the activated complex state and facilitate the reaction.
The activated complex theory explains why not all collisions lead to reactions. Only those collisions that have sufficient energy to overcome the activation energy barrier will result in the formation of product molecules. This theory provides a more accurate understanding of reaction rates and is essential for predicting the outcomes of chemical reactions.
Arrhenius Equation, Eyring Equation, and Transition State Theory: A Deeper Dive into Reaction Kinetics
Chemical reactions are an essential part of our world, but how do we understand their behavior and predict their rates? That’s where reaction kinetics comes in, and three key equations – the Arrhenius equation, the Eyring equation, and transition state theory – play a crucial role. Let’s dive into each one:
Arrhenius Equation
In the late 19th century, Svante Arrhenius proposed an equation that describes how temperature affects reaction rates. The Arrhenius equation states that the rate constant k is exponentially related to the temperature T:
k = Ae^(-Ea/RT)
Where:
- A is the pre-exponential factor (a constant)
- Ea is the activation energy (representing the energy barrier the reaction must overcome)
- R is the universal gas constant
- T is the absolute temperature in Kelvin
The Arrhenius equation is widely used to predict how reaction rates will change with temperature.
Eyring Equation
Later, in the 1930s, Henry Eyring proposed a more sophisticated equation that expanded on the Arrhenius equation. The Eyring equation takes into account the entropic and enthalpic contributions to the activation energy:
k = (kBT/h)e^(-ΔG‡/RT)
Where:
- kB is the Boltzmann constant
- h is Planck’s constant
- ΔG‡ is the free energy of activation (a combination of enthalpy and entropy changes)
The Eyring equation provides a more detailed understanding of the activation process and is often used in more complex reaction systems.
Transition State Theory
Transition state theory is a theoretical model that describes the activated complex, a high-energy unstable intermediate state that forms during a reaction. According to this theory, the rate of a reaction is determined by the free energy barrier that must be overcome to reach this transition state.
By combining the concepts of the Arrhenius equation, the Eyring equation, and transition state theory, we gain a comprehensive understanding of how reaction rates depend on temperature and other factors. These equations are invaluable tools in chemical analysis and provide the foundation for predicting and manipulating chemical reactions in various fields of science and industry.
Free Energy of Activation and the Rate-Determining Step: Unlocking the Secrets of Chemical Reactions
Imagine a bustling city filled with countless individuals, each representing a chemical reactant. They move and interact, but only a few are destined to find their perfect match and form a new molecule. This serendipitous encounter is governed by a hidden force called the free energy of activation.
The free energy of activation is the energy barrier that molecules must overcome to transform into new substances. Think of it as a mountain that reactants must climb before they can reach the summit of a chemical reaction. The higher the mountain, the slower the reaction.
The Rate-Determining Step: A Tale of Patience and Persistence
In the intricate dance of chemical reactions, there’s always a key move that governs the pace of the entire performance. This crucial step, known as the rate-determining step, is the slowest step in the reaction mechanism.
Consider a chemical reaction with multiple steps, like a relay race. Each runner represents a step, and the slowest runner determines the overall speed of the race. Analogously, in a chemical reaction, the rate-determining step sets the pace for the entire transformation.
Unraveling the Reaction Mechanism: A Map to the Molecular Labyrinth
To fully understand a chemical reaction, we need to decipher its intricate choreography, known as the reaction mechanism. This map outlines the sequential steps involved, including the rate-determining step.
By unraveling the reaction mechanism, scientists can predict the products, determine the kinetic parameters, and identify potential catalysts to accelerate or decelerate the reaction.
The Importance of Rate Constant Calculations: A Guide to Reaction Kinetics
Understanding the concepts of free energy of activation and rate-determining step is crucial for calculating rate constants, the numerical values that quantify the speed of chemical reactions. These constants are essential tools for predicting reaction times, optimizing industrial processes, and unraveling the mysteries of complex chemical systems.
By mastering these concepts, scientists and researchers can gain a deeper understanding of chemical reactivity and harness the power of kinetics to unlock the secrets of matter and energy transformations.
Reaction Mechanisms: Demystifying the Dance of Chemical Transformations
In the realm of chemical reactions, understanding the intricate steps involved is crucial to unraveling the mysteries of how substances undergo change. Enter reaction mechanisms, the detailed pathways that describe the series of events leading to the formation of products from reactants.
Imagine a chemical reaction as a graceful dance, where molecules collide, rearrange, and break apart in a carefully choreographed sequence. The reaction mechanism provides a blueprint for this dance, specifying the individual steps, the order in which they occur, and the intermediates that form along the way.
Unveiling the Secrets of Reaction Mechanisms
Delving into the study of reaction mechanisms is like deciphering a coded message. By analyzing experimental data, scientists can deduce the most probable sequence of events that leads to the observed products. The key to unlocking this code lies in understanding the rate-determining step, the slowest step in the reaction mechanism. This step ultimately dictates the overall rate of the reaction.
Rate Constants: Quantifying the Dance
The concept of rate constants plays a pivotal role in unraveling reaction mechanisms. These constants describe the speed at which each step in the mechanism occurs, providing valuable insights into the dynamics of the reaction. By carefully measuring the rate of the reaction under different conditions, scientists can determine the rate constants for each step, allowing them to construct a detailed picture of the reaction mechanism.
The Power of Rate Constants
The knowledge of reaction mechanisms and rate constants empowers chemists with the ability to predict the behavior of chemical reactions under various conditions. This understanding enables them to optimize reaction conditions, design new reactions, and gain a deeper appreciation of the intricate dance of chemical transformations.
Reaction mechanisms are the blueprints of chemical reactions, providing a detailed understanding of how molecules interact and transform. By unraveling these mechanisms and quantifying the rates of individual steps, scientists can gain invaluable insights into the dynamics of chemical processes. This knowledge unlocks the potential for manipulating reactions, predicting their outcomes, and ultimately harnessing their power for advancements in science and technology.
Temperature Dependence of Rate Constant
Temperature plays a crucial role in determining the speed of chemical reactions. The Arrhenius and Eyring equations provide a quantitative understanding of this temperature dependence.
The Arrhenius equation relates the rate constant (k) to temperature (T) and two constants: the pre-exponential factor (A) and the activation energy (Ea):
k = A * exp(-Ea/RT)
Where R is the universal gas constant.
The activation energy represents the minimum energy that reactants must possess to overcome the energy barrier and proceed to the transition state. A higher activation energy results in a slower reaction rate.
The Eyring equation offers a more refined description of the temperature dependence by incorporating the concepts of transition state theory:
k = (kT/h) * exp(-ΔG‡/RT)
Where k is the Boltzmann constant, h is Planck’s constant, and ΔG‡ is the free energy of activation.
As temperature increases, the Boltzmann factor (kT/h) increases, leading to a faster reaction rate. Simultaneously, the exponential term decreases due to the negative activation energy, causing a slower reaction rate.
The balance between these two opposing effects determines the overall temperature dependence of the rate constant. If the increase in the Boltzmann factor outweighs the decrease in the exponential term, the reaction rate will increase with temperature. Conversely, if the decrease in the exponential term dominates, the reaction rate will decrease with temperature.
Understanding the temperature dependence of rate constants is essential in predicting the behavior of chemical reactions in various settings. It enables us to control reaction rates by manipulating temperature and design experiments and processes accordingly.
Concentration Dependence of Rate Constant
In chemical reactions, concentration plays a crucial role in determining the rate at which the reaction proceeds. The rate constant, a value that quantifies the reaction rate, depends not only on the temperature but also on the concentration of the reactants and products involved.
Reactant Concentration:
The concentration of reactants directly influences the frequency of effective collisions between molecules, hence affecting the reaction rate. When the concentration of reactants increases, more molecules are available to collide, leading to a higher probability of successful collisions and a faster reaction rate.
Product Concentration:
In some reactions, the concentration of products can also impact the reaction rate. This effect is particularly significant in reversible reactions, where products can react to form reactants. As the concentration of products increases, the reverse reaction becomes more favorable, resulting in a decrease in the forward reaction rate.
Stoichiometry and Molecularity:
The stoichiometry of a reaction, the balanced chemical equation, provides information about the number of reactant and product molecules involved. The molecularity of a reaction, the number of molecules that participate in the rate-determining step, also affects the concentration dependence.
For unimolecular reactions, where only one molecule is involved in the rate-determining step, the reaction rate is independent of concentration. In bimolecular reactions, involving two molecules, the reaction rate is directly proportional to the concentration of both reactants. For termolecular reactions, involving three molecules, the reaction rate is proportional to the cube of the concentration of the reactants.
Understanding the concentration dependence of rate constant is essential for predicting and controlling reaction rates in various chemical processes, including industrial synthesis, drug development, and environmental remediation. By manipulating the concentration of reactants and products, scientists can fine-tune the reaction rate to optimize desired outcomes.