Calculate Water Mass: A Comprehensive Guide
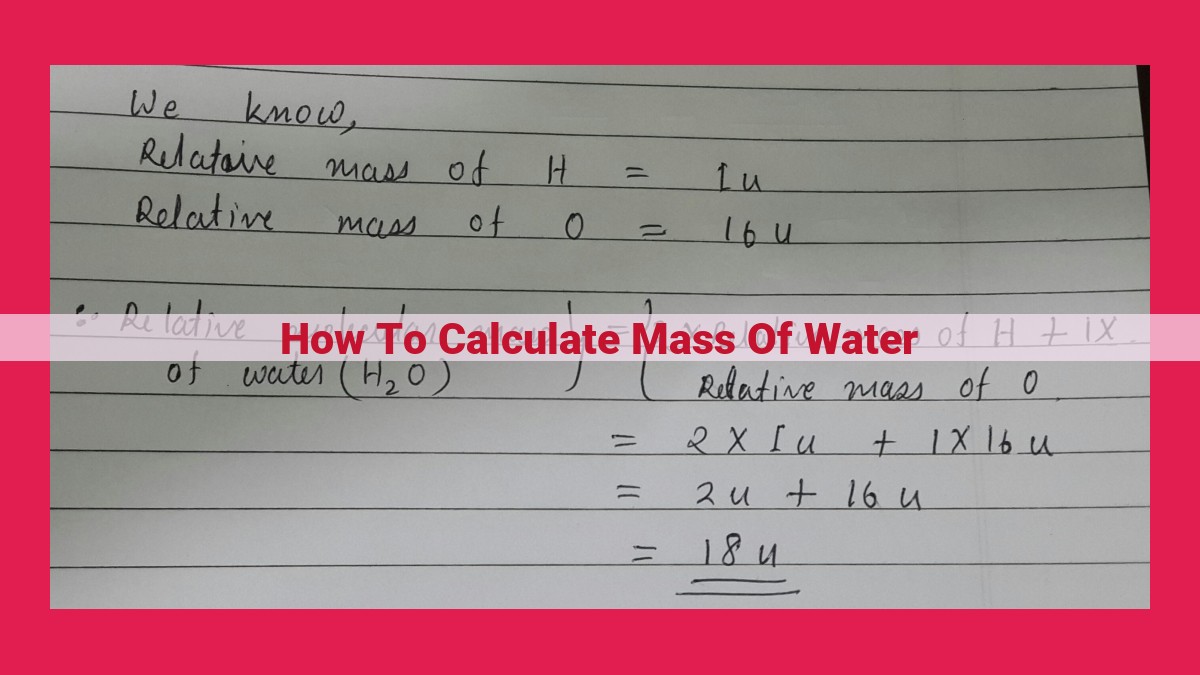
To calculate water mass, understand density (mass per unit volume), and measure water volume. Using the constant water density (1 g/cm³ at 4°C), rearrange the volume equation to derive the mass equation: Mass = Density × Volume. Substitute known values to solve for mass. Convert units if necessary (1 kg = 1000 g; 1 L = 1000 cm³). The formula is: Mass = Density × Volume.
Understanding the Essence of Water’s Density
In the realm of science, density reigns supreme as a fundamental property that governs the behavior of substances. Embark on a captivating journey as we unveil the mysteries of water’s density and explore its profound significance.
Defining the Attributes of Density
Imagine a vast expanse filled with an ethereal substance—mass, representing the intrinsic matter within an object. Its counterpart, volume, depicts the extent of space occupied by that substance. Density emerges as the enigmatic fusion of these two entities:
Density = Mass per Unit Volume
Commonly expressed in units such as grams per cubic centimeter (g/cm³)—or kilograms per liter (kg/L) for larger volumes—density provides a metric to compare the compactness of different substances.
Delving into the Volume of Water
Envision a pristine body of water, its volume embodying the space it occupies within its container. Density and volume embark on a symbiotic dance, with the density of water itself acting as a constant at 4°C (~1 g/cm³). This remarkable stability allows us to deduce volume through a simple equation:
Volume of Water = Mass of Water / Density of Water
Unveiling the Mass of Water
The elusive mass of water remains shrouded in mystery. Yet, our trusty formula empowers us to unravel this enigma:
Mass of Water = Density of Water × Volume of Water
By deftly plugging in known values, we can illuminate the hidden mass of water, revealing its true nature.
Navigating the Labyrinth of Units
The vast scientific landscape teems with diverse units, including grams and kilograms for mass and g/cm³ and kg/L for density. To traverse this labyrinth, we arm ourselves with conversion factors:
- 1 kg = 1000 g
- 1 L = 1000 cm³
These trusty tools guide us through the maze of units, ensuring seamless conversion between different measurement systems.
Applying the Fundamental Equation
Like a master chef orchestrating a culinary symphony, we wield the equation:
Mass of Water = Density of Water × Volume of Water
In a practical setting, let’s imagine a vessel brimming with 500 cm³ of water. Employing our equation, we gracefully calculate:
Mass of Water = 1 g/cm³ × 500 cm³ = 500 g
And thus, the mass of water is revealed in its full glory!
Determining the Volume of Water: A Journey Through Mass, Density, and Constant Values
Understanding Volume: A Tale of Space Occupied
When we talk about the volume of water, we’re referring to the amount of space it takes up. Think of it as the three-dimensional container that houses the water molecules. Volume is measured in cubic units, such as cubic centimeters (cm³) or liters (L).
The Density-Volume-Mass Triangle
Density, mass, and volume are like three sides of a triangle, always interconnected. Density is the mass of an object per unit volume. In other words, it tells us how much matter (mass) is packed into a given amount of space (volume). The unit of density is typically grams per cubic centimeter (g/cm³) or kilograms per liter (kg/L).
The Constant Density of Water: A Temperature-Dependent Equilibrium
The density of water is not constant throughout all temperatures. However, at a specific temperature of 4°C, water reaches its maximum density of approximately 1 g/cm³. This constant density is a valuable property that we can use to determine the volume of water.
The Magic Equation: Unlocking Volume from Mass and Density
Now, let’s unveil the mathematical equation that connects these concepts:
Volume of Water = Mass of Water / Density of Water
This equation allows us to calculate the volume of water if we know its mass and density. By plugging in known values, we can solve for the volume.
Calculating the Mass of Water: An Essential Measurement
Understanding water’s density and its relationship with mass and volume is crucial for various scientific and everyday applications. Mass represents the amount of matter in an object, while volume is the space it occupies. The density of water is defined as its mass per unit volume and is typically expressed in grams per cubic centimeter (g/cm³) or kilograms per liter (kg/L).
One of the most common uses of density is to calculate the mass of water in a given volume. This can be easily achieved using the formula:
Mass of water = Density of water × Volume of water
where the density of water is a constant 1 g/cm³ at 4°C. This means that 1 cubic centimeter of water at this temperature weighs 1 gram.
For example, if you have a container with 2 liters of water, you can calculate its mass using the formula:
Mass of water = 1 g/cm³ × 2 L × (1000 cm³/1 L)
= 2000 g
Therefore, the mass of the 2 liters of water is 2000 grams.
It’s important to note that when working with different units of measurement, conversions are necessary. For instance, to convert from grams to kilograms, divide by 1000 or multiply by 0.001. To convert from cubic centimeters to liters, divide by 1000 or multiply by 0.001.
Understanding the relationship between density, mass, and volume is essential for accurate measurements and plays a vital role in fields such as chemistry, physics, and engineering.
Understanding Water Density: A Guide to Measuring Mass and Volume
In the realm of science, density plays a crucial role in understanding the properties of matter. It’s a measure of how tightly packed the particles are in a substance and is expressed as mass per unit volume. For water, density plays a vital role in many natural phenomena, including the Earth’s water cycle.
Determining Water Volume:
Volume is the amount of space that an object occupies. In the case of water, its volume can be determined using the constant density of water at 4°C, which is approximately 1 g/cm³. This means that 1 gram of water occupies 1 cubic centimeter of space.
Calculating Water Mass:
Mass is the quantity of matter in an object. To calculate the mass of water, we use the formula:
Mass of water = Density of water × Volume of water
For example, if you have 200 cm³ of water, its mass would be:
Mass = 1 g/cm³ × 200 cm³ = 200 g
Converting Units of Measurement:
In scientific measurements, it’s often necessary to convert units. For example, mass can be expressed in grams (g) or kilograms (kg), while volume can be measured in cubic centimeters (cm³) or liters (L).
Conversion Factors:
- 1 kg = 1000 g
- 1 L = 1000 cm³
Using these factors, you can convert between different units of measurement. For instance, to convert 500 g to kilograms:
500 g ÷ 1000 g/kg = 0.5 kg
Applying the Basic Equation:
The fundamental equation for water (Mass = Density × Volume) can be a valuable tool for solving real-world problems.
Example:
Suppose you want to find the mass of 10 liters of water at 4°C. Using the equation:
Mass = 1 g/cm³ × (10 L × 1000 cm³/L) = 10,000 g
Therefore, the mass of 10 liters of water is 10,000 grams or 10 kilograms.
By understanding the concepts of density and unit conversion, you can effectively measure the mass and volume of water, which is essential for various scientific and practical applications.
Mastering Water’s Density: A Comprehensive Guide
Understanding Water’s Density
Density, a crucial concept in physics, is defined as the mass per unit volume. In simpler terms, it measures how much matter an object packs into a given space. For water, density is expressed in grams per cubic centimeter (g/cm³) or kilograms per liter (kg/L).
Determining Water’s Volume
Volume, the amount of space occupied by water, is closely related to mass and density. The constant density of water at 4°C (approximately 1 g/cm³) makes it possible to easily determine its volume. The basic formula:
Volume of water = Mass of water / Density of water
Calculating Water’s Mass
Mass, the quantity of matter in water, can be effortlessly calculated using the formula:
Mass of water = Density of water × Volume of water
Simply substitute the known values to solve for the mass of water.
Converting Units of Measurement
Various units are used to measure mass (g, kg) and density (g/cm³, kg/L). Conversion factors help bridge the gap between these units, such as:
- 1 kg = 1000 g
- 1 L = 1000 cm³
Applying the Basic Equation
To solidify your understanding, let’s put the basic equation into practice with a hypothetical scenario. Suppose we have 200 cm³ of water. To calculate its mass, we substitute the values:
Mass of water = 1 g/cm³ × 200 cm³ = 200 g
Therefore, the mass of the water sample is 200 grams. By mastering these concepts and applying them proficiently, you can effortlessly determine the density, volume, and mass of water in various contexts.